5 Essential Tips for Mole to Particle Conversion

Converting moles to particles is a fundamental skill in the study of chemistry, pivotal when understanding reactions at the microscopic level. Whether you're a student learning chemistry for the first time or a seasoned researcher reviewing foundational principles, mastering the conversion between moles and particles is crucial. This blog post will delve into five essential tips that will help you excel in mole to particle conversion, ensuring accuracy and efficiency in your calculations.
1. Understand the Concept of Avogadro’s Number

Avogadro’s number, denoted by the symbol NA, is a cornerstone in the study of matter at the molecular or atomic level. It represents:
- The number of particles in one mole of any substance.
- Approximately 6.022 x 1023 particles.
Here are key points about Avogadro's number:
- It applies to all types of particles, whether atoms, molecules, ions, or electrons.
- This number is a fundamental constant in chemistry and is often used as a conversion factor between moles and particles.
📚 Note: Avogadro's number is not a 'count', but rather a constant that allows us to relate macroscopic amounts to microscopic counts of particles.
2. Practice Dimensional Analysis

Dimensional analysis is an invaluable tool in chemistry for converting units, especially when dealing with moles and particles. Here’s how to use it:
Step-by-Step Guide:
- Identify your starting and target units: You'll start with moles and want to end with particles or vice versa.
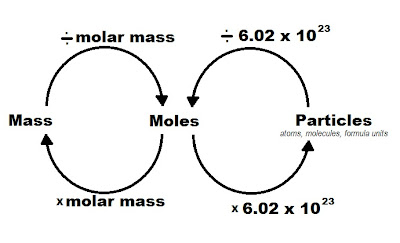
- Use Avogadro's number as your conversion factor: Since 1 mole equals 6.022 x 1023 particles, you can set up your equation as follows:
- To convert moles to particles: moles x (6.022 x 1023 particles/mole)
- To convert particles to moles: particles ÷ (6.022 x 1023 particles/mole)
- Perform the calculation: Ensure to multiply or divide as necessary based on the direction of your conversion.
- Check your units: The final result should have the units you need (particles or moles).
🔬 Note: Pay attention to the direction of your conversion. Mistakes are common when converting from particles to moles since you must divide by Avogadro's number, not multiply.
3. Use Molar Mass for Molecule Counting

When dealing with compounds, understanding molar mass helps in converting grams to moles to particles. Here’s how:
Steps for Conversion:
- Calculate the molar mass: Sum the atomic masses from the periodic table for each element in the compound.
- Convert mass to moles: Use the molar mass as your conversion factor to find the number of moles.
- Convert moles to particles: Apply Avogadro's number to find the number of particles.
| Element | Atomic Mass (g/mol) | Number of Atoms |
|---|---|---|
| Carbon (C) | 12.011 | 1 |
| Hydrogen (H) | 1.008 | 4 |
| Oxygen (O) | 15.999 | 2 |
| Molar Mass of CH3COOH (Acetic Acid) | 60.053 g/mol | |

🧪 Note: If a sample mass is given, first find moles using molar mass before converting to particles.
4. Keep Track of Units

Units matter in chemistry, and ensuring they are consistent throughout your calculations is key to accurate results:
- Moles: Use the mole (mol) unit.
- Particles: Counted in entities such as atoms, molecules, or ions.
- Mass: Use grams (g) or any other mass unit if you’re converting mass to moles to particles.
Example Calculation:
If you have 2 moles of oxygen gas (O2), you can calculate the number of particles as follows:
2 moles O2 x (6.022 x 1023 particles/mole) = 1.2044 x 1024 particles of O2
📝 Note: Always state your final answer with the correct units (e.g., particles, molecules, ions).
5. Understand the Difference Between Avogadro’s Number and Molar Mass

Understanding the distinction between Avogadro’s number and molar mass is crucial for avoiding confusion:
- Avogadro's Number: 6.022 x 1023 entities per mole.
- Molar Mass: The mass of one mole of a substance in grams, which is also the molecular or atomic weight expressed in grams.
Remember:
- Avogadro's number helps you count particles, not weigh them.
- Molar mass relates to mass, which then can be converted to moles for particle counting.
In sum, by embracing these five tips, you'll enhance your proficiency in converting moles to particles. Understanding the importance of Avogadro's number, practicing dimensional analysis, utilizing molar mass, keeping track of units, and distinguishing between related concepts will ensure your conversions are accurate and efficient. This knowledge not only aids in academic performance but is fundamental in practical chemistry applications where quantity matters.
Why is Avogadro’s number so important in chemistry?

+
Avogadro’s number provides a link between the macroscopic scale (moles) and the microscopic scale (particles), allowing chemists to measure substances by counting particles rather than weighing them directly.
Can you give a real-world example of mole to particle conversion?

+
Consider making aspirin from salicylic acid. Chemists would convert the grams of salicylic acid to moles, then to the number of particles (molecules), to ensure the correct amount for reaction with acetic anhydride.
What if I want to convert particles back to moles?

+
To convert particles to moles, you divide the number of particles by Avogadro’s number. For example, if you have 3.011 x 10^23 particles, divide by 6.022 x 10^23 to get 0.5 moles.
Does the substance’s state affect mole-particle conversions?
+
No, the physical state does not change the number of moles or particles in a given mass of substance, although it might affect how you measure or handle the substance.
Why do I need to use molar mass in some conversions?

+
Molar mass serves as a bridge between mass and moles, enabling you to convert grams of a substance into moles, which can then be converted into particle counts.