5 Steps to Solving Mole Ratio Problems Easily
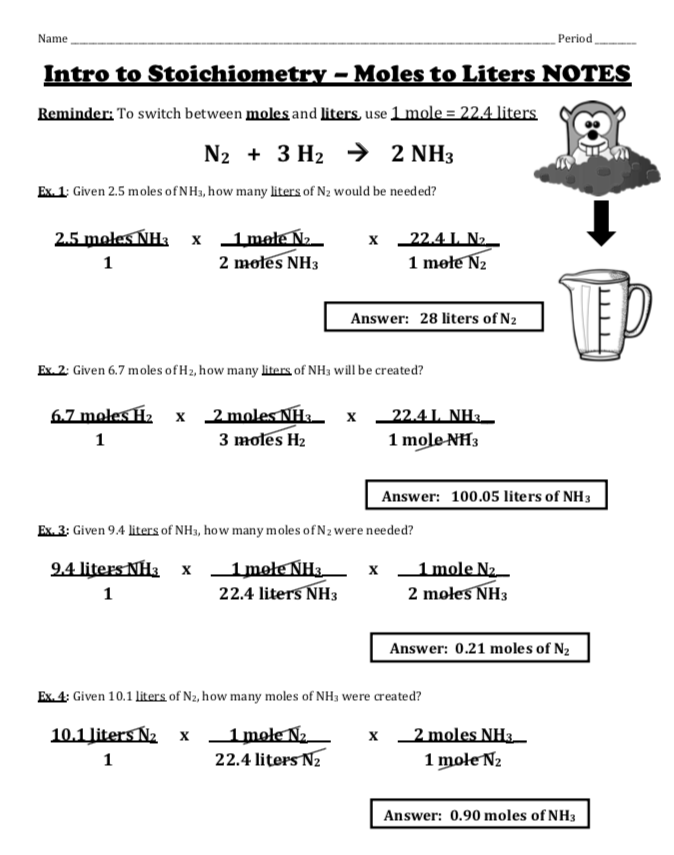
In the world of chemistry, the mole ratio is a fundamental concept that acts as a bridge between the macroscopic world and the microscopic realm of atoms and molecules. For students, grappling with mole ratios can be a daunting task. However, with a clear understanding of the process, these problems can be solved systematically and efficiently. In this guide, we'll delve into five essential steps to tackle mole ratio problems, ensuring you can approach them with confidence.
Step 1: Understanding the Balanced Chemical Equation

A balanced chemical equation tells you the proportion in which reactants combine to form products. Here are key points to consider:
- Ensure the equation is balanced. If not, balance it first.
- Identify the substances involved in the reaction.
- Highlight the coefficients in front of each reactant and product as they represent the mole ratios.
Step 2: Determine the Mole Ratio from the Equation

Once you have a balanced equation, you can establish the mole ratios:
- Read the coefficients as the number of moles involved in the reaction.
- The ratio of these coefficients provides the mole ratio for the given equation.
Let’s consider an example for a better understanding:
| Equation | 2H₂ + O₂ → 2H₂O |
|---|---|
| Coefficients | 2 : 1 : 2 |
| Mole Ratio | 2 moles of H₂ react with 1 mole of O₂ to produce 2 moles of H₂O |

Step 3: Calculate the Moles of Reactants or Products
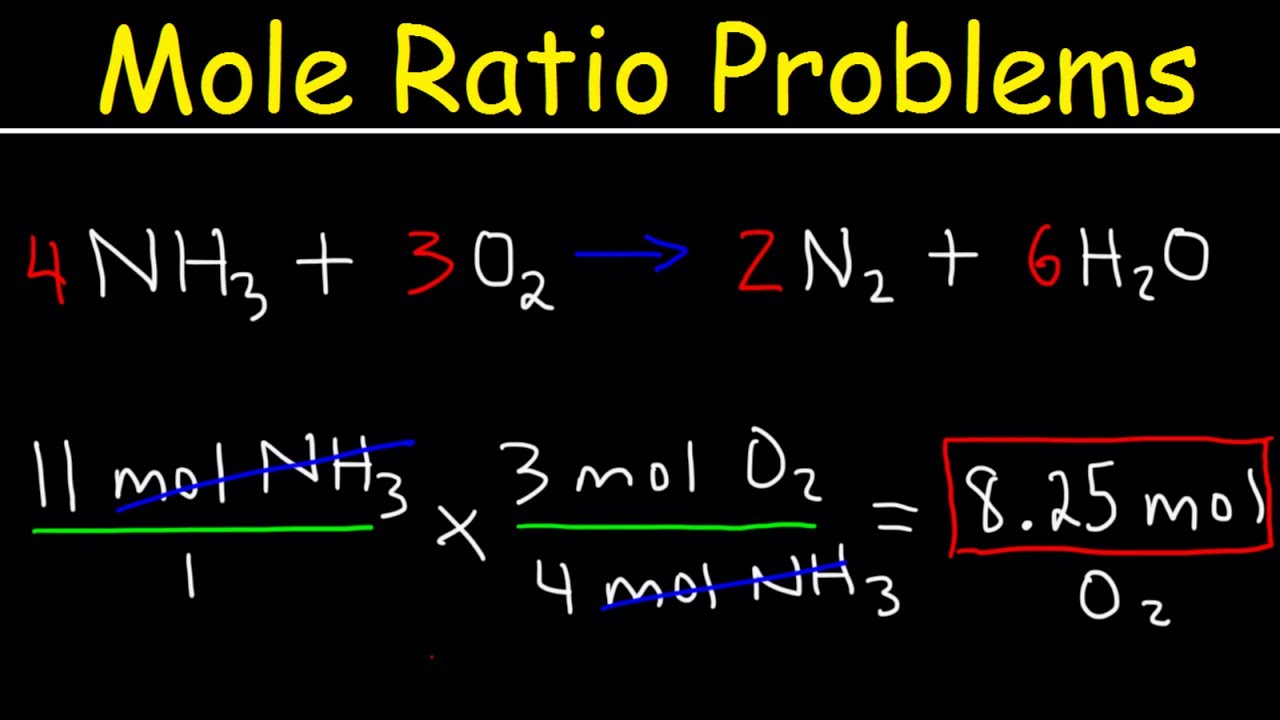
To convert from grams to moles or vice versa, use the molar mass:
- Given the mass of a substance, convert it to moles by dividing by its molar mass.
- Using the mole ratio, determine how many moles of other substances are involved.
For example:
🔬 Note: Make sure to convert all masses to moles before applying the mole ratio.
Step 4: Use Mole Ratio to Find the Unknown

With moles of one substance known, use the mole ratio to find the moles of other substances:
- Set up a proportion using the mole ratio.
- Solve for the unknown variable.
Here’s an equation to help:
unknown moles = given moles * (mole ratio of unknown to given)
Step 5: Convert Moles to Grams if Necessary

After finding the moles of the substance you are interested in:
- Multiply the moles by the molar mass of the substance to convert to grams.
Remember, practice makes perfect. Mole ratio problems require patience and familiarity with stoichiometry. Here are a few final notes to help you along:
🧪 Note: Always double-check your work. A common mistake is incorrect balancing or misapplying the mole ratio.
Embarking on your chemical journey, mastering mole ratios allows you to interpret reactions at the molecular level. You now possess a structured method to solve these problems with precision. Keep practicing, and soon these calculations will become second nature. Whether you're converting substances in a lab experiment or understanding chemical processes in textbooks, you're now equipped to handle mole ratio calculations effectively. This understanding not only enhances your grasp of chemistry but also provides a practical tool for everyday problem-solving in the chemical world.
What is a mole ratio in chemistry?

+
A mole ratio is the proportion in which reactants combine to form products based on the coefficients of a balanced chemical equation.
Why is it important to use a balanced chemical equation for mole ratio calculations?

+
A balanced equation ensures that the law of conservation of mass is obeyed, and it provides the correct ratios between the substances involved in the reaction.
Can you solve a mole ratio problem if you only know the mass of one reactant?

+
Yes, you can convert the mass of the reactant into moles using its molar mass, then use the mole ratio from the balanced equation to find the moles of other substances involved in the reaction.