Master Mole to Gram Conversion: Simple Worksheet Guide

Converting between moles and grams is a foundational concept in chemistry, pivotal for understanding reactions and the quantitative aspects of chemical processes. Whether you're a student, teacher, or simply a curious mind, this guide will walk you through the simple steps of performing mole to gram conversions. Let's delve into this crucial chemistry conversion with a step-by-step approach.
The Basics of Mole to Gram Conversion

To understand mole to gram conversion, you first need to grasp a few key concepts:
- Mole: A mole is a unit representing 6.022 x 10^23 entities (atoms, molecules, ions, etc.)
- Molar Mass: The mass of one mole of a substance, typically expressed in grams per mole (g/mol)
- Avogadro’s Number: The number of entities in one mole, which is 6.022 x 10^23
These are the building blocks for our conversions:
Step-by-Step Guide to Converting Moles to Grams

Here’s how you can convert moles to grams using a simple worksheet approach:
- Identify the Substance: Know which compound or element you’re working with.
- Find the Molar Mass: Determine the molar mass of the substance in g/mol. This can be found using a periodic table or calculated from atomic masses.
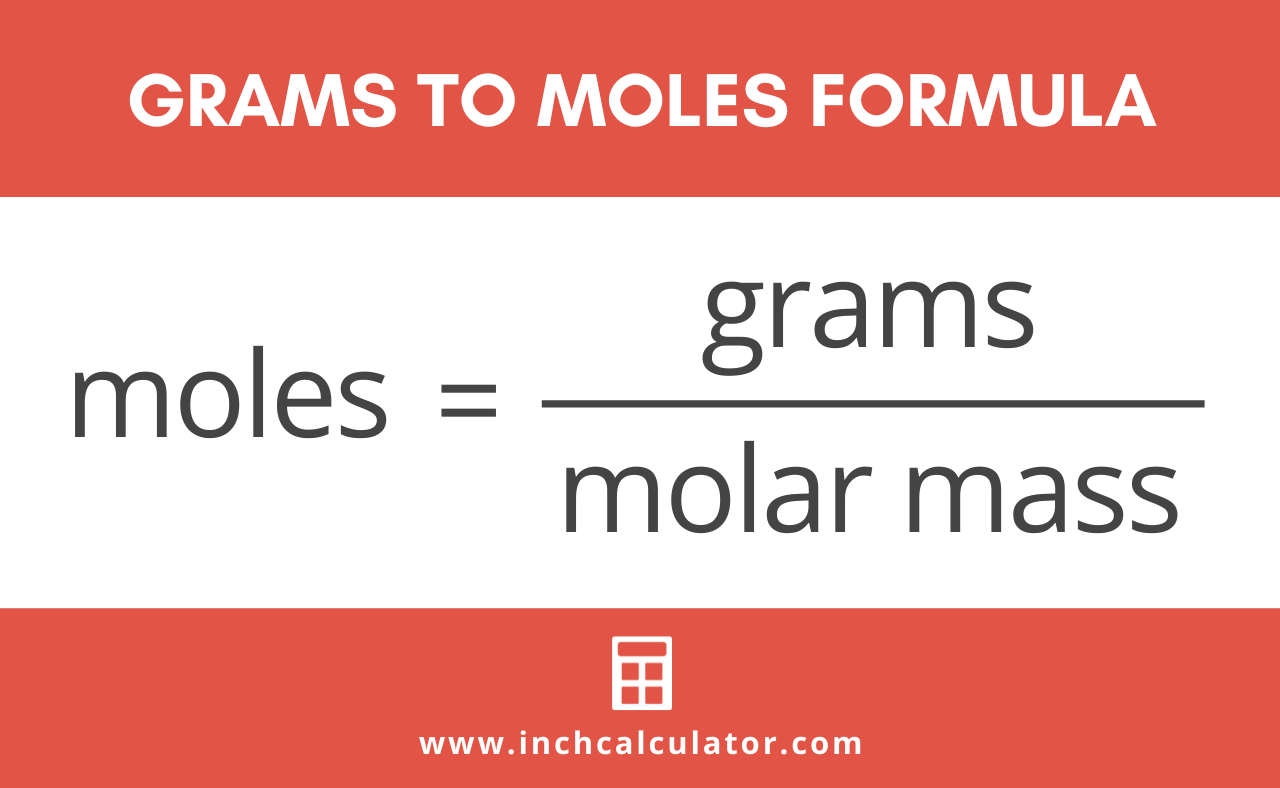
- Set Up Your Equation: The equation for this conversion is straightforward: Grams = Moles x Molar Mass.
- Plug in the Values: Multiply the number of moles by the molar mass to get the mass in grams.
💡 Note: Ensure you're using the correct units. Molar mass must be in g/mol and moles should be unitless or as moles.
Example Conversion

Let’s work through an example to cement our understanding:
Suppose you have 2 moles of CO2. How many grams is this?
- Identify the substance: Carbon Dioxide (CO2)
- Find the Molar Mass:
Element Atomic Mass Carbon © 12.01 g/mol Oxygen (O) 16.00 g/mol x 2 = 32.00 g/mol CO2 12.01 + 32.00 = 44.01 g/mol 
- Set Up Your Equation:
Grams = Moles x Molar Mass
- Plug in the Values:
Grams = 2 moles x 44.01 g/mol = 88.02 g
Practical Applications

Understanding mole to gram conversions has real-world applications:
- Pharmaceutical dosing: Accurate dosing depends on molecular mass calculations.
- Environmental Analysis: Quantifying pollutants or greenhouse gases in the atmosphere.
- Food Science: Understanding the nutritional content by analyzing the composition of food at the molecular level.
Common Pitfalls

Here are some mistakes to watch out for:
- Not Rounding: Molar masses can be rounded to two decimal places for simplicity.
- Unit Confusion: Mixing up units like mg or kg with g/mol.
- Not Considering Hydration: For ionic compounds, hydrates have water molecules attached, affecting the molar mass.
⚠️ Note: Conversion errors can lead to significant mistakes in experiments or calculations. Double-check your math and units!
Tips for Successful Conversions
To master mole to gram conversions:
- Use a Periodic Table: Keep a periodic table handy for quick access to atomic masses.
- Check Your Work: Always recompute your calculations to ensure accuracy.
- Practice: Regular practice makes the process automatic and reduces errors.
- Memorize Common Molar Masses: Familiarize yourself with common substances for quicker calculations.
Summing Up

Understanding how to convert between moles and grams is indispensable for anyone engaging with the molecular world of chemistry. It’s not just about numbers; it’s about understanding the essence of substances, their compositions, and interactions. With this guide, you’re equipped to navigate these conversions with ease, whether in a lab, classroom, or home study. Remember, mastering this skill opens doors to deeper chemical explorations and practical applications in science.
Why do we need to know the molar mass for mole to gram conversions?

+
The molar mass provides a direct link between moles and grams. It represents how much mass (in grams) one mole of a substance has, allowing us to convert moles to mass or vice versa.
Can I use the same molar mass for all substances?
+
No, each substance has a unique molar mass based on the atomic masses of its constituent elements and their quantities in the molecular structure.
What should I do if my calculation result is too high or too low?

+
Recheck your calculation for common mistakes like rounding errors, incorrect molar mass, or unit conversion issues. Consider using scientific notation for large numbers to avoid errors.