5 Essential Tips for Mole Calculation Mastery

Embarking on the journey of mastering mole calculation can be both exciting and daunting. Whether you're a chemistry student, a teacher, or simply a science enthusiast, understanding how to perform mole calculations is fundamental to grasping the essence of chemistry. This long-form guide will walk you through the intricacies of moles, offering 5 essential tips that will transform you into a mole calculation wizard. So, let's dive into the world of particles, moles, and chemical reactions!
Understanding the Mole

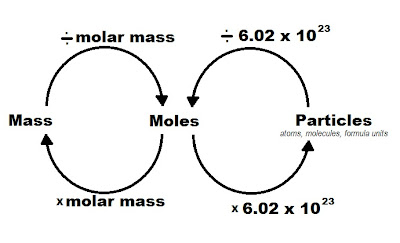
Before diving into the calculations, let's first establish what a mole is. In chemistry, a mole is a unit that quantifies the number of particles in a substance. Just like a dozen represents 12 items, a mole represents 6.022 x 10^23 entities, known as Avogadro's number. Here’s how you can understand the mole:
- Definition: A mole is the amount of a substance that contains the same number of particles (atoms, molecules, ions, or any other entities) as there are atoms in 12 grams of carbon-12.
- Avogadro's Number: This is the key to mole calculations; it represents the number of entities in one mole, which is approximately 6.022 x 10^23.

Tip 1: Master the Mole-Mass Relationship

One of the first steps in mastering mole calculations is understanding the relationship between the mass of a substance and its amount in moles. Here's how you can do it:
- Molar Mass: Calculate the molar mass (in grams per mole, g/mol) of the compound using its molecular formula. This involves adding up the atomic masses of all atoms in one molecule of the substance.
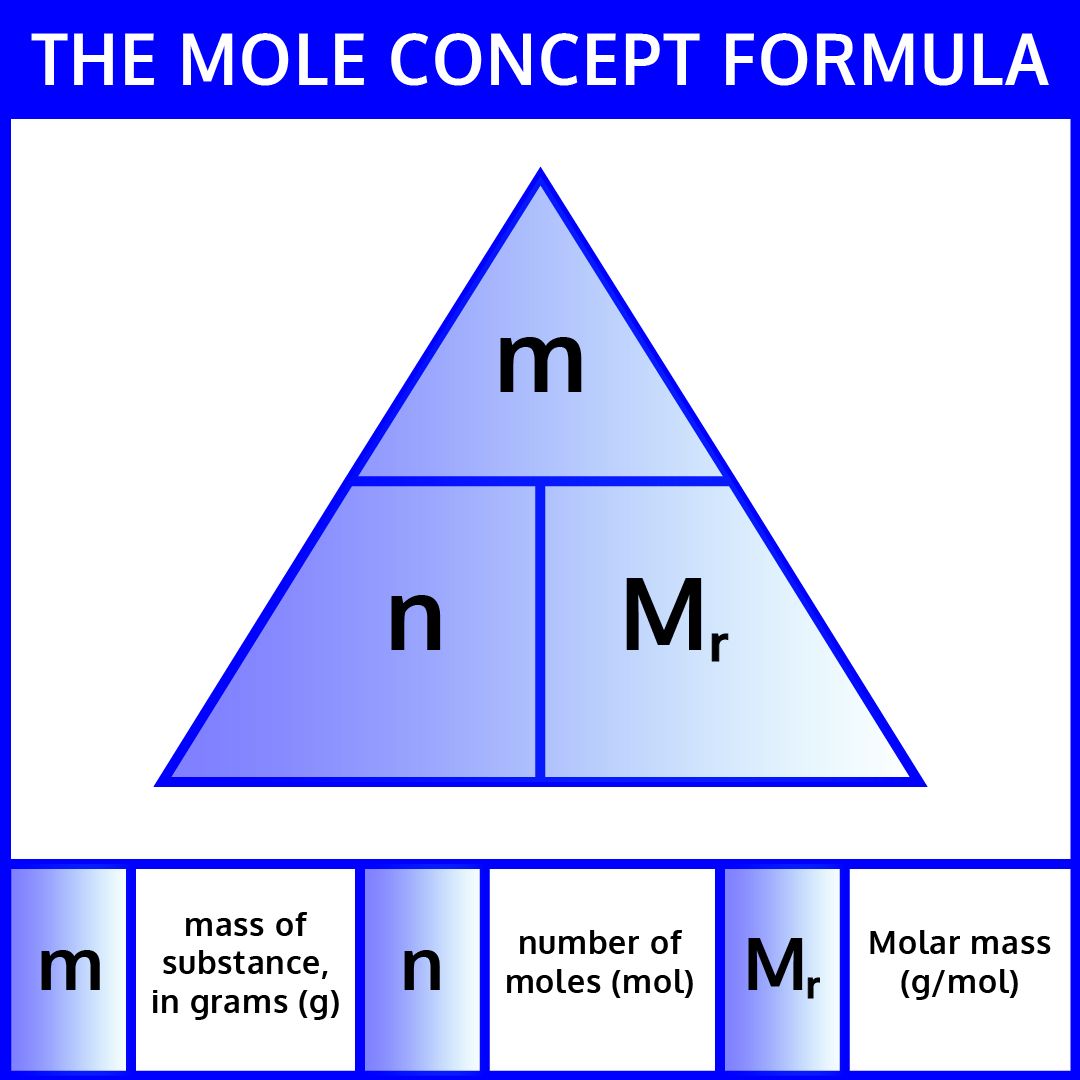
- Formula: Use the formula \[ \text{Number of Moles} = \frac{\text{Mass of Substance (g)}}{\text{Molar Mass (g/mol)}} \] to convert from mass to moles.
🔧 Note: Always ensure your periodic table is up-to-date as atomic masses can vary slightly over time with new measurements.
Tip 2: Use Mole Ratio in Chemical Equations

Chemical equations provide a framework for understanding how different substances interact in reactions. Here are key points to consider:
- Stoichiometry: Utilize the coefficients in balanced chemical equations as mole ratios. This tells you the quantitative relationship between reactants and products.
- Example: For the reaction 2H_2 + O_2 \rightarrow 2H_2O, the mole ratio of H_2 to O_2 is 2:1, and to H_2O is 2:2 or simply 1:1.
By mastering these ratios, you can predict how much product can form from a given amount of reactants or how much reactant is needed to produce a certain amount of product.
Tip 3: Solve for Limiting Reactants
In many chemical reactions, one reactant will be completely consumed before the others, stopping the reaction. Here's how to identify the limiting reactant:
- Calculate Moles: Convert the quantities of reactants into moles.
- Compare Ratios: Use the mole ratios from the balanced equation to determine which reactant will run out first.
- Example: If you have 4 moles of H_2 and 1 mole of O_2 for the above reaction, O_2 is limiting because it will run out before H_2.
🧐 Note: Always calculate for the excess reactant as well to understand how much of each substance remains after the reaction.
Tip 4: Practice with Complex Reactions

Once you're comfortable with basic mole calculations, expand your skillset with more complex reactions:
- Multi-step Reactions: Break down the overall reaction into smaller, manageable steps. Calculate moles for each step separately.
- Simultaneous Equations: Some reactions might require solving multiple equations at once to find the moles involved.
- Example: Consider the synthesis of ammonia from nitrogen and hydrogen in the Haber process, involving several steps and conditions that affect the yield.
Tip 5: Utilize Online Resources and Tools

To hone your mole calculation skills, leveraging online resources can be beneficial:
- Mole Calculators: Use online tools for quick mole-mass conversions, especially for complex molecules.
- Interactive Simulations: Websites like PhET Interactive Simulations offer visual and interactive ways to learn about moles and reactions.
- Tutorials and Guides: Free educational platforms like Khan Academy or Coursera provide step-by-step guides and explanations.
Why is Avogadro’s number important?

+
Avogadro’s number is crucial as it provides a fixed number of entities (like atoms or molecules) in one mole of any substance, allowing for consistent calculations in chemistry.
What’s the difference between a limiting reactant and excess reactant?

+
The limiting reactant is the substance that is completely consumed first in a chemical reaction, thereby limiting the amount of product formed. The excess reactant is the substance that remains after the reaction stops due to the depletion of the limiting reactant.
How do you convert between mass and moles?

+
Use the formula: [ \text{Number of Moles} = \frac{\text{Mass of Substance (g)}}{\text{Molar Mass (g/mol)}} ]. You need the molar mass of the substance, which can be found using the periodic table and summing the masses of its constituent atoms.
Can the stoichiometry change in a chemical reaction?

+
No, the stoichiometry, or the mole ratios, does not change in a chemical reaction. It is determined by the coefficients in the balanced chemical equation and reflects the law of conservation of mass.
How does temperature and pressure affect mole calculations?
+
Temperature and pressure can affect the physical state of gases and their volume but not the number of moles or the intrinsic properties of chemical reactions. However, for gases, the ideal gas law (PV = nRT) (where (n) is the number of moles) accounts for changes in conditions.