Molarity Worksheet Answers: Your Ultimate Guide
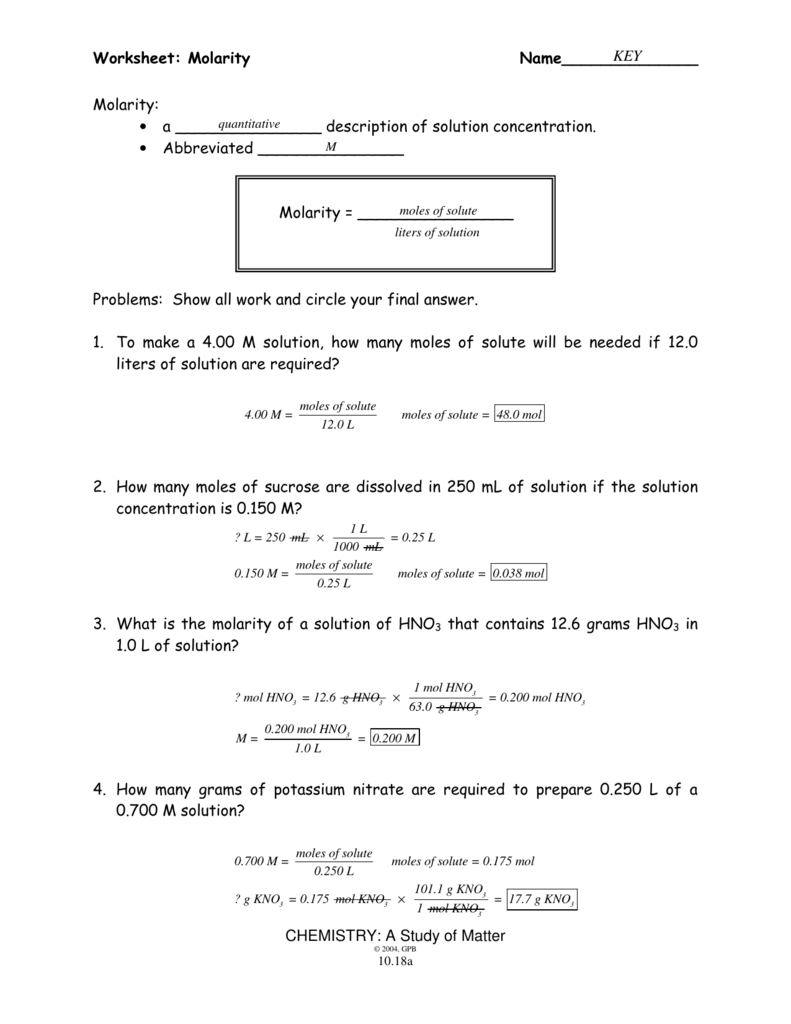
Understanding Molarity: A Comprehensive Guide

Welcome to a comprehensive guide on understanding molarity, one of the fundamental concepts in chemistry that involves the concentration of solutions. Molarity, often denoted as “M,” is crucial in many chemical experiments, industrial processes, and biological systems where precise concentration measurements are essential. This guide will take you through the basic definition, how to calculate molarity, its applications, and practical examples to reinforce your understanding.
What is Molarity?
Molarity is defined as the number of moles of solute dissolved in one liter of solution. Here’s how you can express it:
Molarity (M) = moles of solute / volume of solution in liters
This equation signifies that molarity measures how many moles of a compound are found in a volume of solution. It’s a crucial measure for scientists when preparing solutions for various chemical reactions, ensuring the right concentration for the desired chemical reaction or procedure.
Examples to illustrate:
- If you dissolve 2 moles of sodium chloride (NaCl) in 1 liter of water, the molarity of the solution would be 2 M.
- Dissolving 0.5 moles of sugar in 500 mL (0.5 L) of water results in a molarity of 1 M.
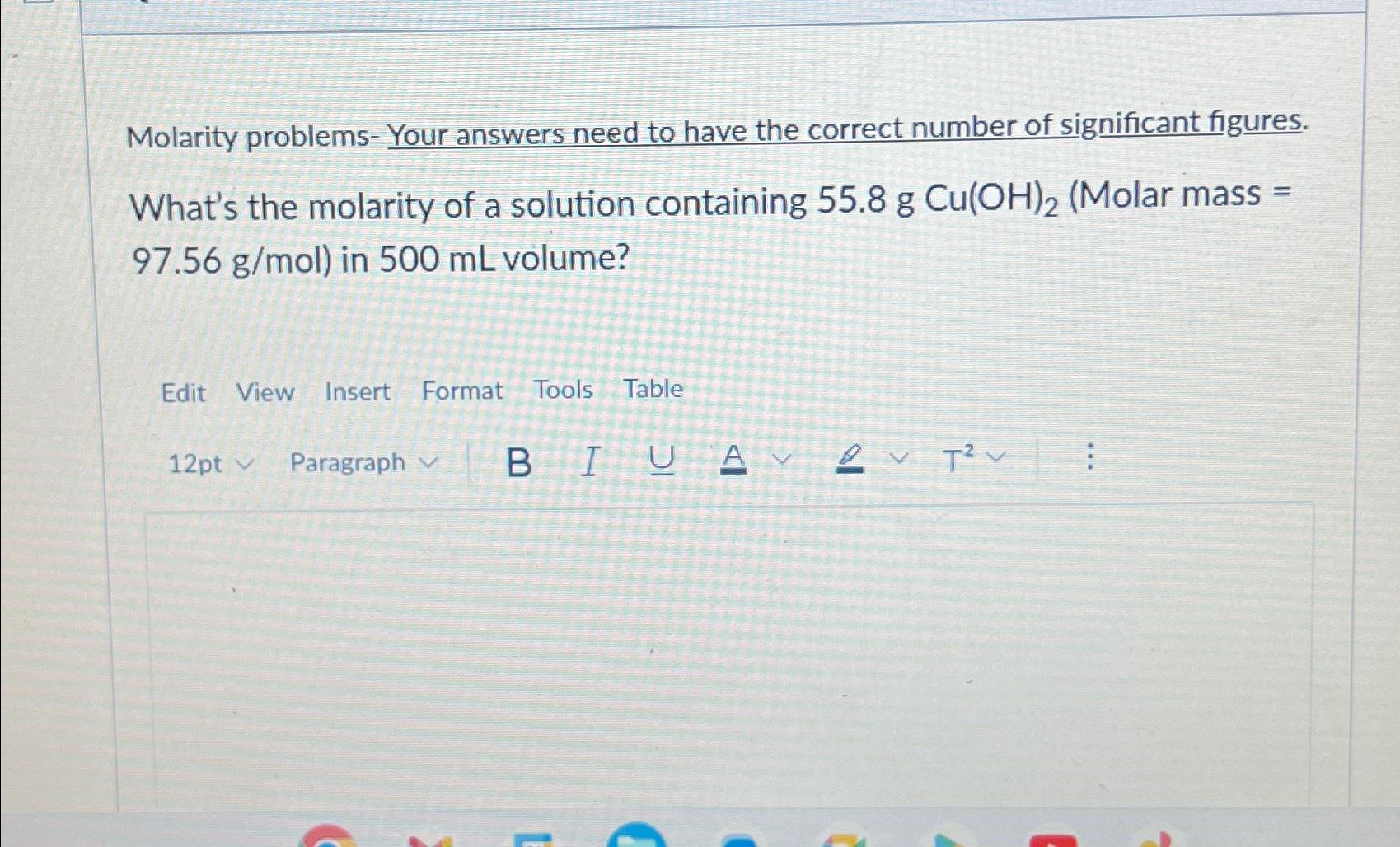
Calculating Molarity: Step by Step

Here’s a detailed breakdown on how to calculate the molarity of a solution:
- Determine the Moles of Solute: This involves knowing the molecular weight of the solute. You can calculate the moles using the formula:
moles = mass of solute (in grams) / molar mass of solute (in g/mol)
- Measure the Volume of the Solution: The volume must be in liters (L). Ensure that if you measure in milliliters (mL), you convert it to liters by dividing by 1000 (1 L = 1000 mL).
- Apply the Molarity Formula: Plug the values into the molarity formula:
M = moles of solute / volume of solution (L)
Let’s work through an example:
Calculate the molarity of a solution made by dissolving 58.5 grams of sodium chloride (NaCl) in 2 liters of water:
- Molar mass of NaCl = 58.5 g/mol
- moles of NaCl = 58.5 g / 58.5 g/mol = 1 mol
- Molarity = 1 mol / 2 L = 0.5 M
Applications of Molarity

Molarity is not just a theoretical concept; it has practical applications in:
- Biochemistry: For enzyme kinetics and other biochemical processes where precise concentration is critical.
- Medicine: In pharmaceuticals where the dosage of drugs in solution form must be accurately measured.
- Environmental Chemistry: For monitoring and treating water pollution, where pollutants must be quantified in molar concentrations.
- Industrial Processes: For reactions where specific concentrations are required for optimal yield and efficiency.
Notes on Molarity Calculations
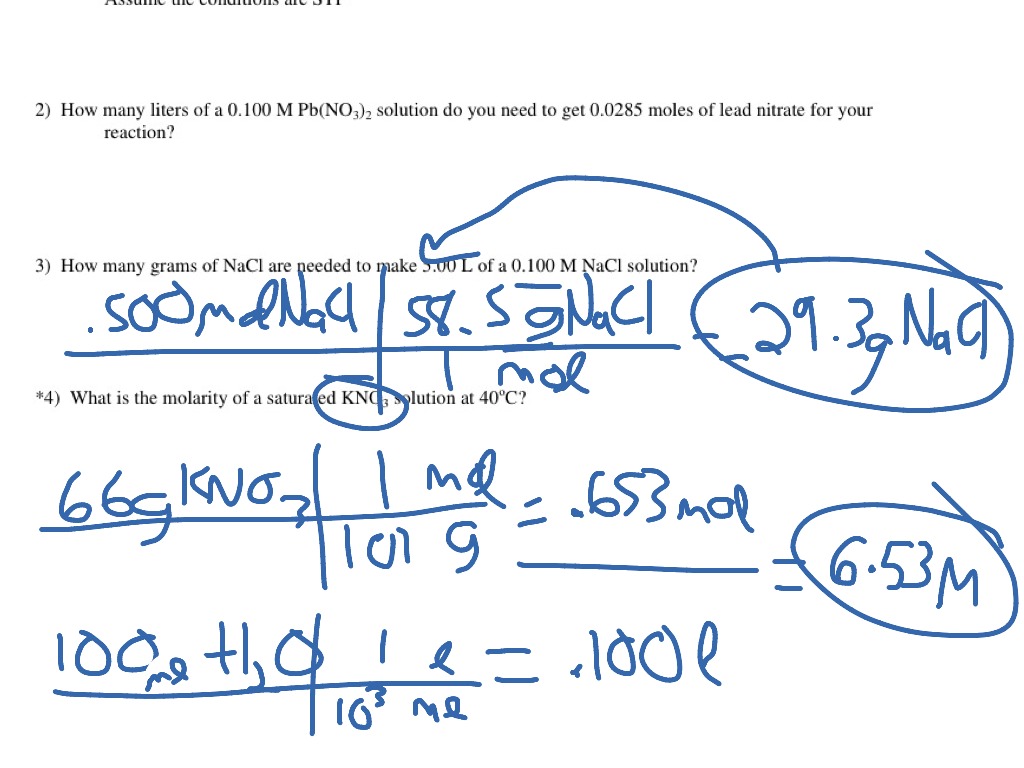
🔍 Note: Remember that the volume used in molarity calculations is the total volume of the solution, not just the solvent. This volume might differ from the volume of solvent due to the solute itself taking up space in the solution.
🔍 Note: Temperature affects molarity because the volume of a solution can change with temperature, impacting the concentration of the solution. Be consistent with the conditions under which you prepare your solution.
Wrapping Up

In summary, understanding molarity is essential for anyone working in or studying chemistry-related fields. It’s the measure that ensures precision in solution preparation, affecting everything from small-scale lab experiments to large-scale industrial processes. The ability to calculate molarity, apply it in various scenarios, and understand its implications is crucial for advancing in chemistry or any science where solution concentration plays a role. Remember, when you’re dealing with molarity, accuracy and consistency in measurements are paramount to ensure experimental or industrial reliability.
Why is molarity an important measure in chemistry?

+
Molarity allows chemists to quantify the concentration of solutions, ensuring consistency in reactions, dosage accuracy in pharmaceuticals, and proper dilution in environmental studies.
How does temperature affect molarity?

+
Temperature affects the volume of the solution, which in turn changes the molarity. Higher temperatures generally increase the volume, potentially decreasing the molarity if the moles of solute remain constant.
Can molarity be used for all types of solutes?

+
Yes, molarity can be used to express the concentration of any solute that can dissolve in a solvent, whether it’s a solid, liquid, or gas, as long as the solute dissociates into moles.