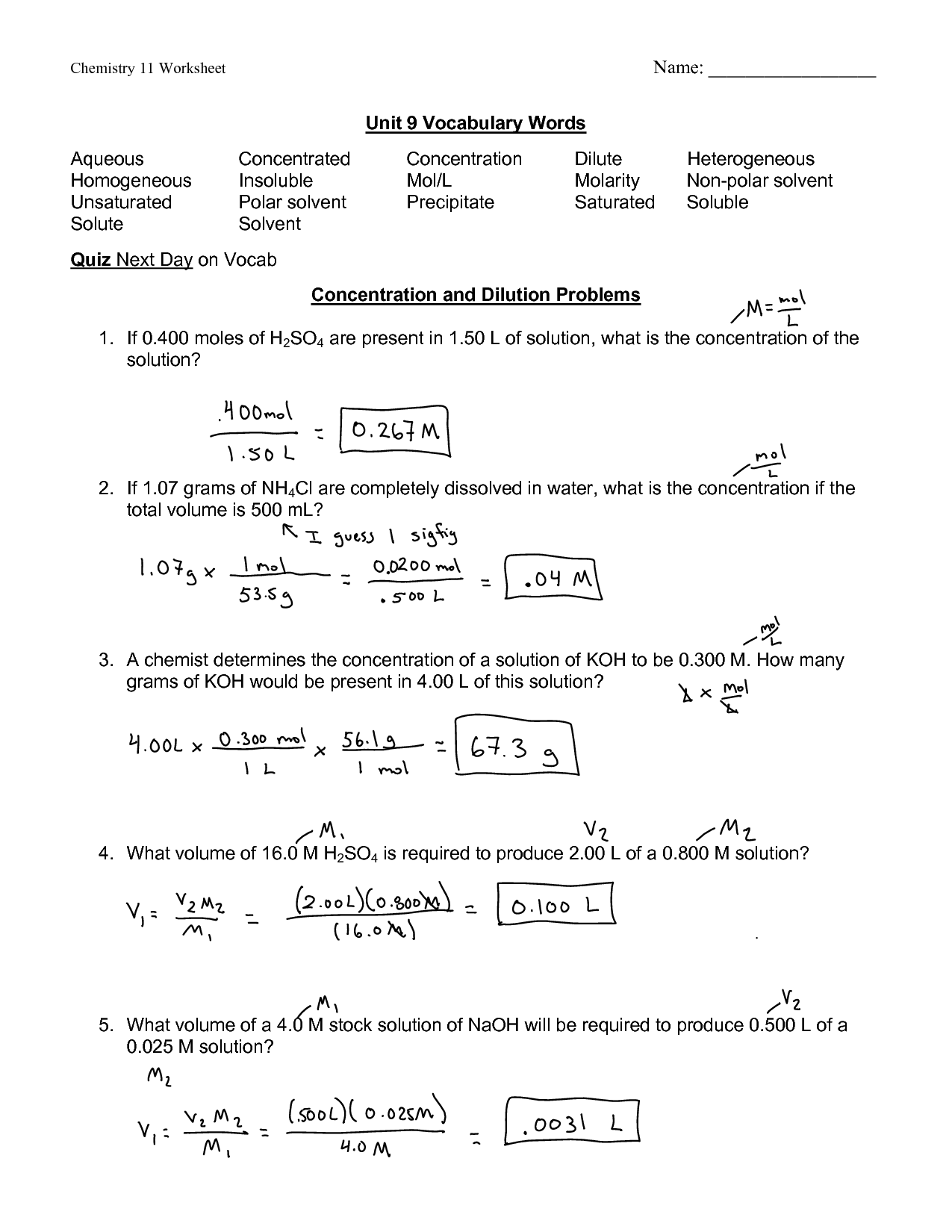
Molarity M Worksheet Answers: Simplify Chemistry with These Solutions

Understanding Molarity

Molarity, often represented with the symbol 'M', is a common measurement in chemistry that quantifies the concentration of a solute in a solution. It is defined as the number of moles of solute per liter of solution. Understanding molarity is essential for students studying chemistry, as it provides a foundation for various lab experiments and calculations. This worksheet aims to demystify the concept of molarity by presenting questions with step-by-step solutions to help you grasp this fundamental idea.
Calculating Molarity: The Basics

To calculate molarity, you need to know the following:
- Number of moles (mol): This is calculated from the mass of solute and its molar mass.
- Volume of solution (L): Measured or determined after solute and solvent are mixed.
Here’s the basic formula for molarity:
Molarity (M) = moles of solute / volume of solution (in liters)
Let's dive into some examples:
Example 1: Sodium Chloride (NaCl) Solution

Question: How do you prepare a 250 mL solution of sodium chloride with a molarity of 0.10 M?
Solution:
- Find the molar mass of NaCl:
- Na: 23.0 g/mol
- Cl: 35.5 g/mol
- Molar mass = 23.0 + 35.5 = 58.5 g/mol
- Calculate moles required:
Moles = Molarity × Volume (L) Moles = 0.10 M × 0.250 L Moles = 0.025 mol - Determine mass of solute:
Mass = Moles × Molar mass Mass = 0.025 mol × 58.5 g/mol Mass = 1.46 g - To prepare the solution, you need to weigh out 1.46 grams of sodium chloride, dissolve it in a small amount of water, then transfer this to a 250 mL volumetric flask, and finally dilute to the mark.
⚠️ Note: Always remember to mix the solute with a small volume of water before diluting to avoid localized high concentration that might not dissolve properly.
Example 2: Using Given Mass and Volume

Question: What is the molarity of a solution made by dissolving 15.7 grams of potassium nitrate (KNO3) in enough water to make 1.50 liters of solution?
Solution:
- Determine the moles of KNO3:
Molar mass of KNO3 = 39.1 (K) + 14.0 (N) + 3(16.0) (O) = 101.1 g/mol Moles = 15.7 g / 101.1 g/mol Moles ≈ 0.155 mol - Calculate molarity:
Molarity = Moles / Volume (L) Molarity = 0.155 mol / 1.50 L Molarity ≈ 0.103 M
Using Molarity in Dilutions

Dilution is a common practice in chemistry where a concentrated solution is diluted to make a less concentrated one. Here’s how you can approach dilution:
M1 × V1 = M2 × V2
Where:
- M1 = Molarity of the original, more concentrated solution
- V1 = Volume of the original solution
- M2 = Molarity of the final, diluted solution
- V2 = Final volume of the diluted solution
Example 3: Dilution
Question: If you take 50 mL of a 6.0 M sulfuric acid (H2SO4) solution and dilute it to 250 mL, what is the molarity of the resulting solution?
Solution:
6.0 M × 0.050 L = M2 × 0.250 L
M2 = (6.0 × 0.050) / 0.250
M2 = 1.2 M
Conclusion

By working through these examples, you now have a better understanding of how to calculate molarity, both in preparing solutions and in performing dilutions. These concepts are not just theoretical but are practically applied in labs, environmental studies, and even in industries like pharmaceuticals where precise concentrations are critical. Always remember to double-check your units and use the appropriate formula to maintain accuracy in your calculations.
What is molarity and why is it important?

+
Molarity (M) is a measure of the concentration of a solute in a solution, expressed as moles of solute per liter of solution. It’s crucial in chemistry for controlling reactions, determining stoichiometry, and in preparing solutions for experiments.
How do you convert between molarity and moles?

+
Using the formula Molarity = moles / volume, you can calculate moles from molarity by multiplying molarity by volume in liters, or calculate molarity from moles by dividing moles by volume.
Can molarity change with temperature?

+
Yes, because molarity is volume dependent, and volume changes with temperature. An increase in temperature can expand the volume of the solution, potentially decreasing its molarity.
What’s the difference between molarity and molality?

+
Molarity involves the volume of the solution, while molality is based on the mass of the solvent. Molality (m) is moles of solute per kilogram of solvent, and it does not change with temperature.
Why use molarity instead of other concentration units?

+
Molarity is often preferred because it directly relates to the chemical amounts needed for reactions and is easily scalable. It’s also straightforward to prepare solutions of known molarity, which is vital for many chemical processes.