5 Simple Tips to Master Mixtures, Elements, and Compounds

Understanding the fundamental concepts of chemistry can seem daunting at first, but once you get the hang of it, the world of chemicals and reactions opens up in an exciting and comprehensive way. Today, let's delve into some crucial tips to master the differentiation and interaction of mixtures, elements, and compounds.
Tip 1: Understand the Definition


Elements, compounds, and mixtures are the building blocks of matter:
- Elements are substances composed of only one type of atom. They cannot be broken down into simpler substances by chemical means.
- Compounds are substances formed when two or more elements chemically combine in fixed proportions.
- Mixtures, on the other hand, are physical combinations of two or more substances where each substance retains its own chemical identity.
Tip 2: Learn the Language of Chemistry

Familiarity with the periodic table is essential. Here's a basic table showing the first 10 elements:
| Atomic Number | Symbol | Name |
|---|---|---|
| 1 | H | Hydrogen |
| 2 | He | Helium |
| 3 | Li | Lithium |
| 4 | Be | Beryllium |
| 5 | B | Boron |
| 6 | C | Carbon |
| 7 | N | Nitrogen |
| 8 | O | Oxygen |
| 9 | F | Fluorine |
| 10 | Ne | Neon |

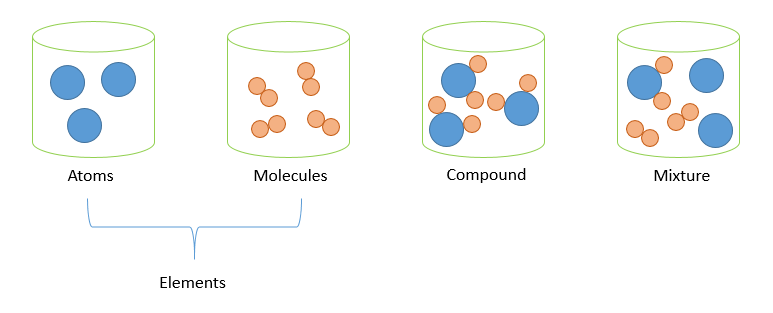
Tip 3: Visualize with Diagrams
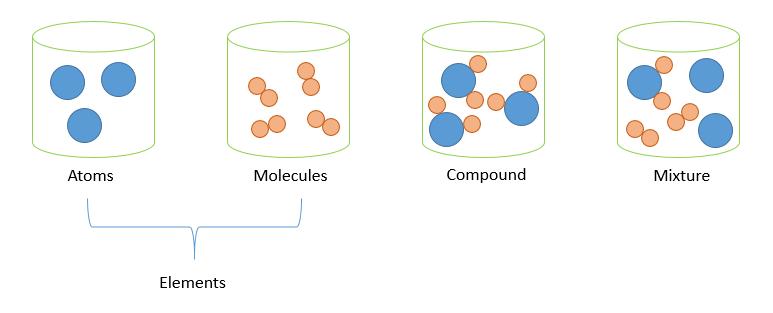

Diagrams can greatly enhance your understanding. Chemical bonding diagrams help you visualize how elements combine to form compounds, which in turn, helps distinguish them from mixtures where components are not bonded.
Tip 4: Experiment and Observe

Conduct simple experiments or observe everyday phenomena:
- Mix salt (NaCl) with water to see how a compound dissolves but retains its chemical identity.
- Observe iron filings and sulfur powder in a mixture - you can see both components separately, but when heated, they form a compound, iron sulfide, with new properties.
⚗️ Note: Be cautious when performing chemical experiments. Always ensure safety measures are in place and follow instructions meticulously.
Tip 5: Use Mnemonic Devices
Memory aids can be incredibly helpful. Here are a few examples:
- “Peter Eats Camels” - Periodic Elements Concept to remember the Periodic Table.
- “MIXed Identities” - Mixtures retain individual properties.
To summarize, mastering mixtures, elements, and compounds involves understanding their definitions, familiarizing yourself with chemical symbols and bonding, visualizing through diagrams, gaining hands-on experience, and using memory aids. This knowledge not only enriches your understanding of the world around you but also opens doors to higher-level scientific study and applications.
What’s the difference between a mixture and a compound?

+
Mixtures are combinations of two or more substances where each substance retains its own chemical identity, and they can be easily separated through physical means like filtration or distillation. Compounds, however, are formed when elements chemically combine in fixed ratios, and they have properties distinct from their constituent elements, requiring chemical reactions to separate.
How do I remember the periodic table?

+
Using mnemonics can be very effective. For instance, “Peter Eats Camels” to remember the first three elements, or creating songs or rhymes with the symbols. Regularly referring to the periodic table and associating each element with its common compounds or reactions can also reinforce memory.
Can elements be broken down further?
+
Elements are considered the simplest form of matter that cannot be broken down into simpler substances by ordinary chemical means. However, through nuclear reactions, atoms can be split or combined to form different elements.