5 Steps to Master Limiting Reagents Worksheet Key
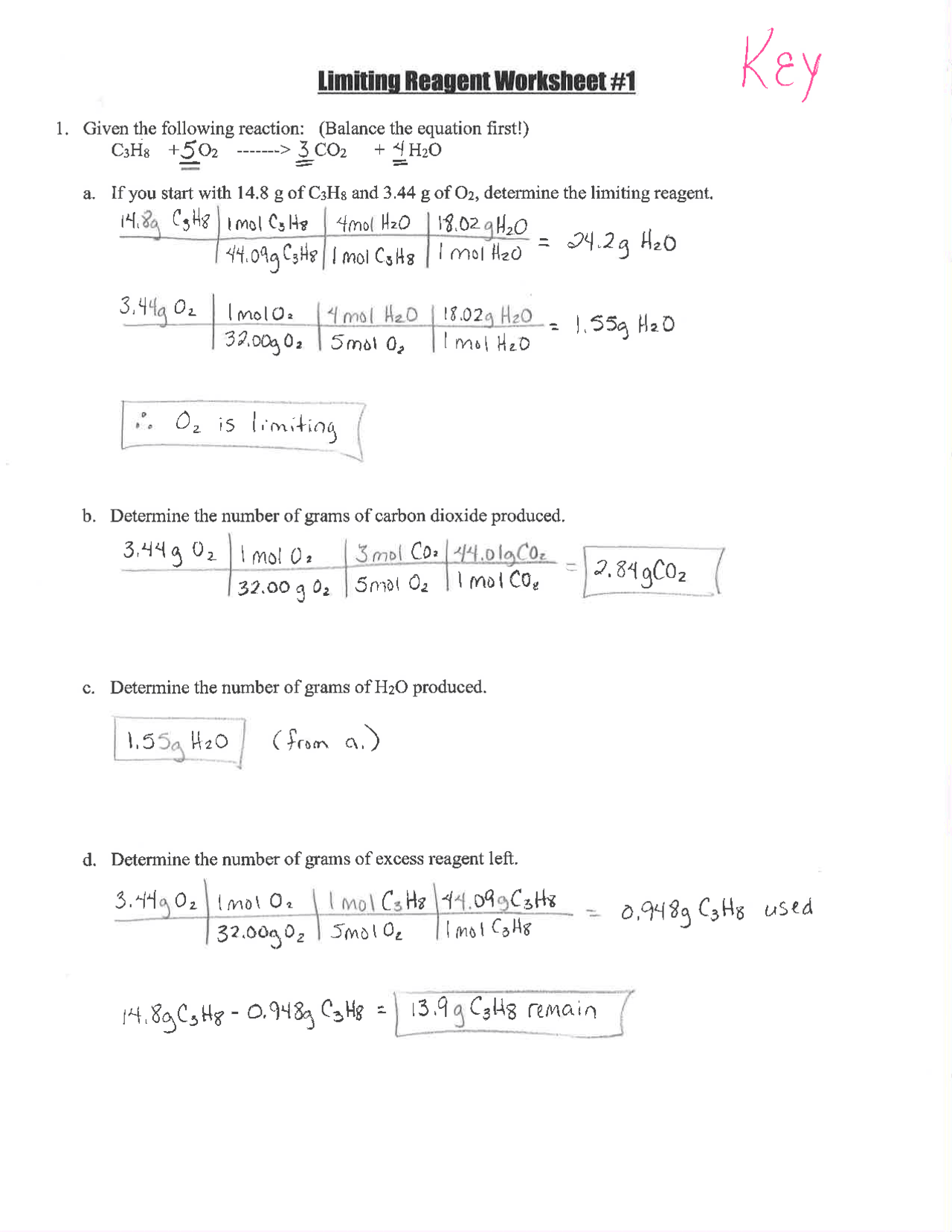
Limiting reagents are a fundamental concept in stoichiometry, crucial for any student looking to excel in chemistry, especially in reaction calculations and problem solving. Understanding how to identify and calculate with limiting reagents is essential for accurately predicting the outcomes of chemical reactions. This post will outline a detailed, step-by-step guide to mastering the limiting reagents worksheet key, ensuring you can confidently tackle these problems.
Step 1: Define Limiting and Excess Reagents

Before diving into calculations, it’s pivotal to grasp what we mean by limiting and excess reagents:
- Limiting Reagent: This is the reactant that is entirely consumed in a chemical reaction, thereby determining how much product can form.
- Excess Reagent: This reactant is present in quantities greater than necessary, meaning it will not be fully consumed.
Understanding these terms sets the foundation for your calculations.
Step 2: Write the Balanced Chemical Equation

A balanced chemical equation is your roadmap for the entire problem-solving process. Ensure you:
- Write out the chemical equation
- Balance it to ensure the law of conservation of mass is upheld.
Balancing ensures that the ratio of moles of reactants to products is correct, which is key to all subsequent calculations.
Step 3: Calculate Moles of Reactants

Using the molecular weight or the given mass, convert the mass of each reactant into moles:
- Formula for moles: moles = mass / molecular weight
This step allows you to compare the moles of reactants directly with the balanced equation.
🔍 Note: When dealing with gases or solutions, use volume or concentration respectively instead of mass.
Step 4: Determine the Limiting Reagent

To find the limiting reagent:
- Divide moles of each reactant by the stoichiometric coefficient from the balanced equation.
- The reactant with the smallest ratio is the limiting reagent.
This comparison is vital as it tells you which reactant will control the reaction’s progress.
Step 5: Calculate the Product Amount
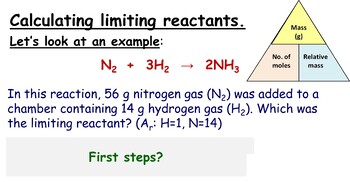
Once you’ve identified the limiting reagent, you can now calculate how much product will be formed:
- Use the moles of the limiting reagent to determine the moles of product using the stoichiometry from the balanced equation.
- If needed, convert the moles back to mass using the molecular weight of the product.
This calculation represents the theoretical yield of the reaction.
⚠️ Note: The actual yield can vary due to inefficiencies or side reactions, so be cautious in interpreting these results in experimental contexts.
In the pursuit of mastering chemistry, understanding and applying the concept of limiting reagents is a critical skill. By following the five steps outlined in this post, you can confidently determine which reactant will be limiting, calculate the amount of product formed, and comprehend the efficiency of a chemical reaction. Remember, practice is key; working through worksheets and real-world scenarios will hone your ability to identify and work with limiting reagents effectively.
What’s the difference between limiting and excess reagents?

+
The limiting reagent is the substance that gets used up first in a chemical reaction, thus limiting the amount of product that can form. Excess reagents are those reactants that are available in quantities greater than necessary for the reaction, meaning not all of it will be used up.
Why do we balance chemical equations?

+
Balancing chemical equations ensures the law of conservation of mass is observed, meaning that the number of atoms of each element is the same on both sides of the equation. This balance is critical for understanding how much of each reactant is needed and how much product can form.
How do I know which reagent is limiting?

+
To determine the limiting reagent, you compare the moles of each reactant to the stoichiometric coefficients from the balanced equation. The reactant that has the smallest ratio after this comparison is the limiting reagent.