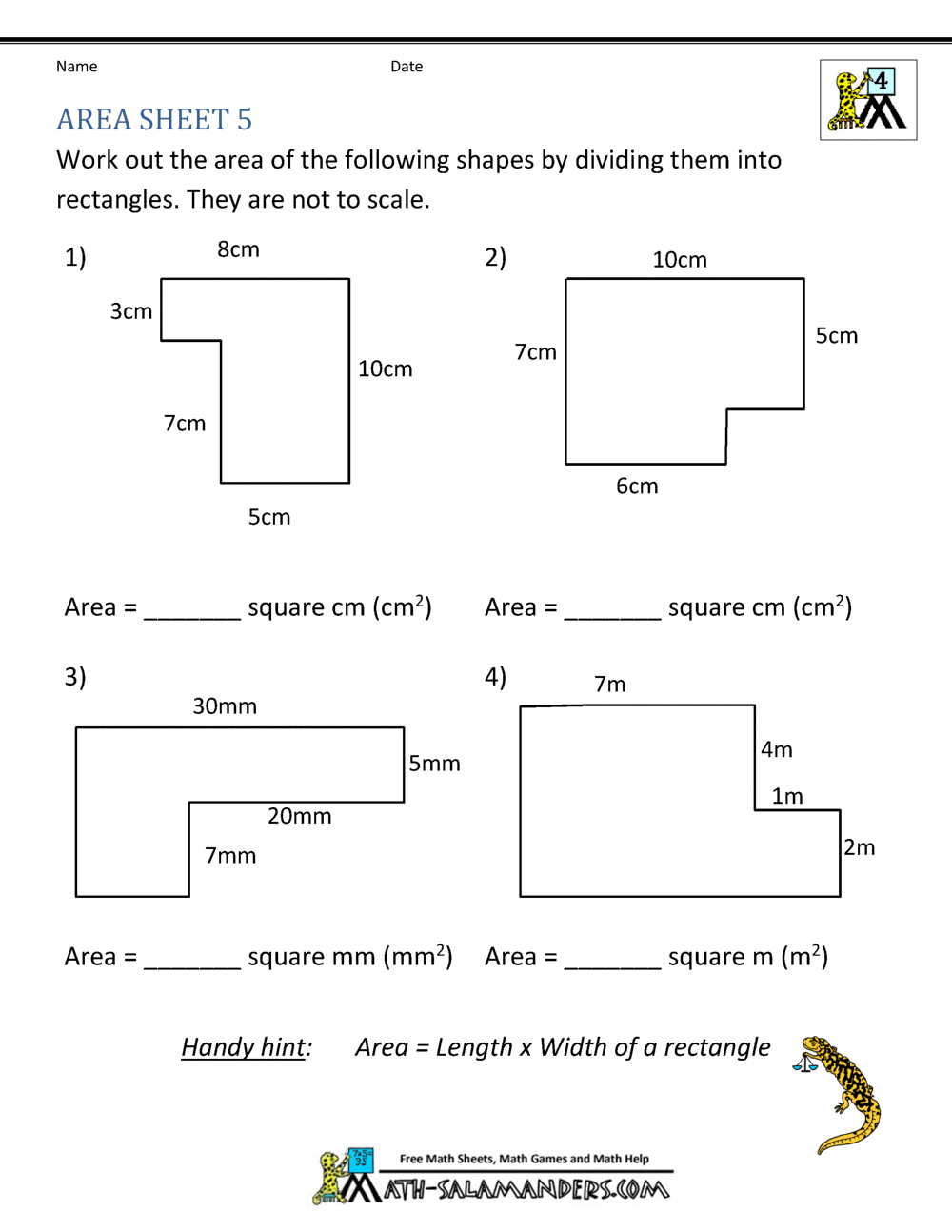
5 Steps to Identify Limiting Reagent Quickly

In the world of chemistry, understanding the limiting reagent is fundamental when conducting experiments that involve chemical reactions. The limiting reagent is the reactant that gets completely consumed first, dictating the amount of products formed and essentially limiting the extent of the reaction. Here's a comprehensive guide to identifying the limiting reagent swiftly and effectively.
Step 1: Write the Balanced Chemical Equation
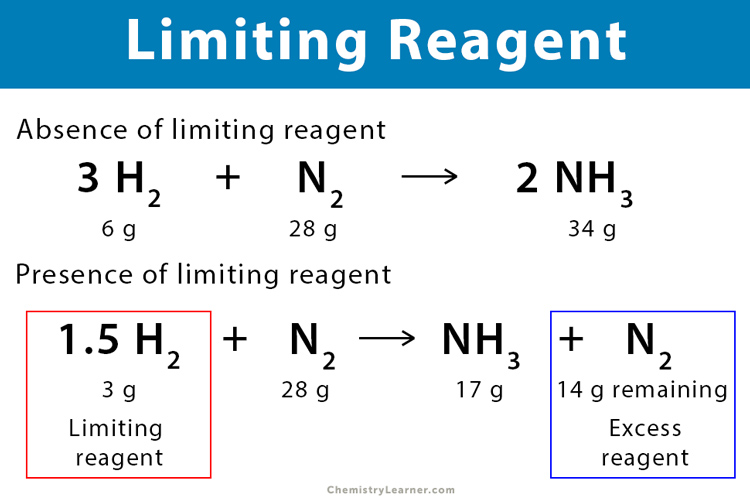
Before you can identify the limiting reagent, you need a clear and balanced chemical equation:
- Ensure that the equation is balanced, meaning the number of atoms of each element is equal on both sides of the reaction arrow.
- This provides the stoichiometric ratio between reactants, which is crucial for further calculations.
⚗️ Note: Balancing a chemical equation ensures conservation of mass; each atom of reactant must appear in the product.
Step 2: Determine the Moles of Each Reactant

With your balanced equation in hand:
- Convert the given mass or volume of each reactant into moles using their respective molecular weights or molar volumes.
- If the reactants are gases at STP, use the molar volume of gas (22.4 L at STP is 1 mole).
Step 3: Use Stoichiometry to Find Reactant Ratios

Now, refer back to your balanced equation:
- Calculate the amount of product that could theoretically be produced if each reactant were the limiting reagent, using the mole ratios from the equation.
- For example, if the equation is: A + 3B → 2C, and you have 1 mole of A and 4 moles of B, you could calculate 2 moles of C if A were limiting and 2.67 moles if B were.
Step 4: Identify the Limiting Reagent
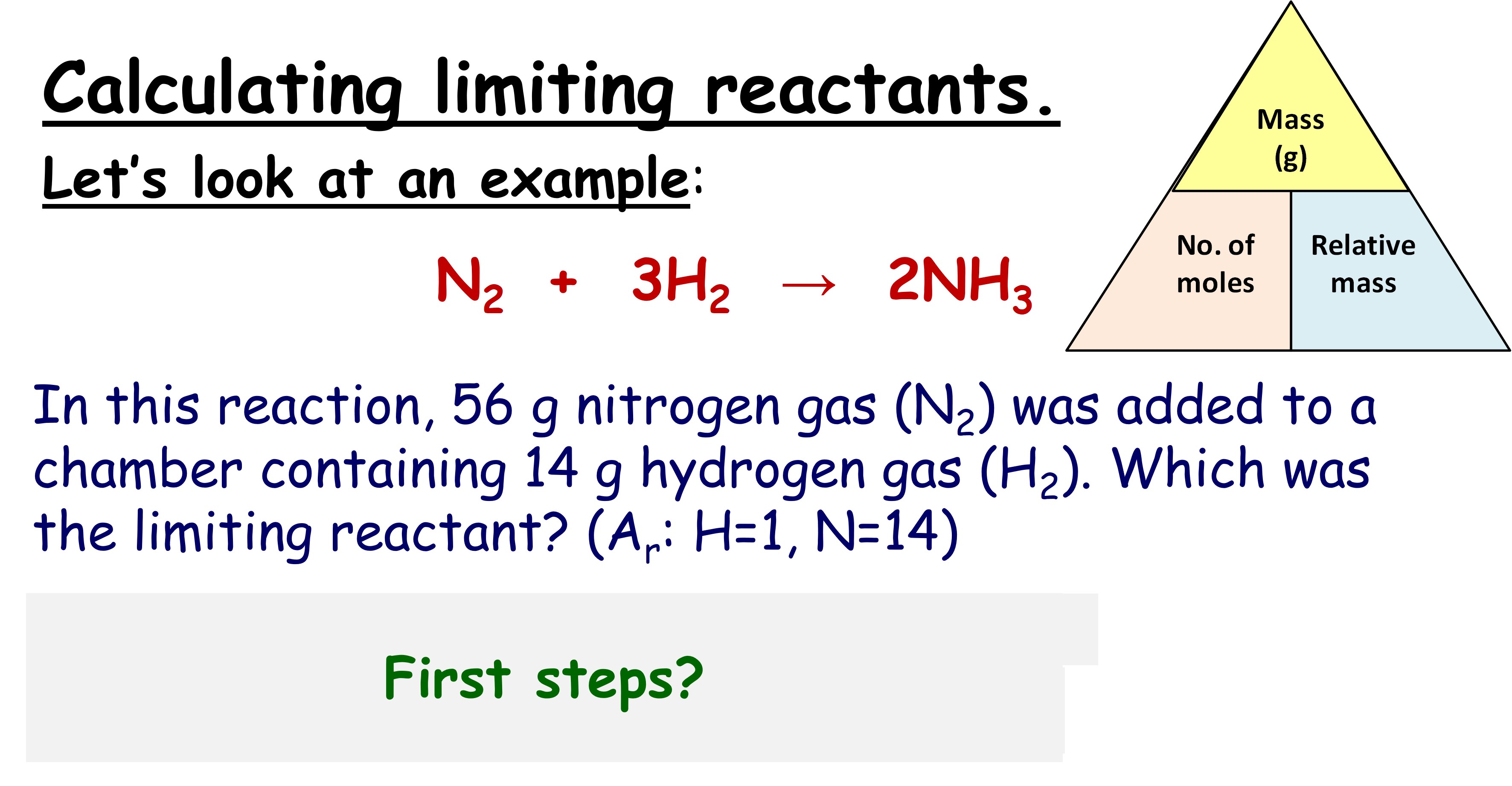
To find which reactant will run out first:
- The reactant that produces the least amount of product based on your calculations in Step 3 is your limiting reagent.
- Another method is to divide the moles of each reactant by its stoichiometric coefficient; the smallest ratio is the limiting reagent.
| Reactant | Moles | Stoichiometric Coefficient | Ratio |
|---|---|---|---|
| A | 1 | 1 | 1 |
| B | 4 | 3 | 1.33 |

📌 Note: The ratio method also helps in understanding the excess reagent, which is the reactant present in a greater amount than needed.
Step 5: Calculate the Theoretical Yield

With the limiting reagent identified:
- Use the moles of the limiting reagent and the balanced equation to calculate the maximum amount of product (theoretical yield) that can be produced.
- Compare this with experimental yield to understand reaction efficiency.
To ensure that you accurately pinpoint the limiting reagent in your chemistry experiments, consider these final notes:
- Always double-check your calculations; mistakes in stoichiometry can lead to incorrect conclusions.
- Understand that in real-world reactions, factors like solubility, temperature, and pressure might influence the actual yield.
🔍 Note: If any reactant is in excess, the yield of the reaction will not be affected by its quantity as long as the limiting reagent's amount remains the same.
Understanding the concept of limiting reagents allows chemists to predict reaction outcomes more accurately, ensuring efficient use of resources and optimal production of desired compounds. By following these steps, you'll not only become adept at identifying the limiting reagent but also enhance your overall grasp of chemical reactions.
What if my reactants are not given in moles?

+
Convert the given mass or volume to moles by dividing the mass by the molecular weight or using the molar volume for gases at STP.
Can a limiting reagent change during a reaction?

+
While theoretically, the limiting reagent remains constant, in practical scenarios, if a reactant is added in stages or if reaction conditions change significantly, the limiting reagent could shift.
What if I can’t identify the limiting reagent easily?

+
If the difference between the theoretical yields is small or if calculations seem confusing, perform a careful mole ratio analysis. Sometimes, reviewing your work or seeking a second opinion can help clarify.
Why is understanding the limiting reagent important in industrial processes?

+
In industry, knowing the limiting reagent helps in optimizing costs, reducing waste, and ensuring that production runs smoothly without excess reactant waste.
How do I deal with limiting reagents in a multistep synthesis?
+
In multistep synthesis, identify the limiting reagent for each step individually, as the product from one step might become the limiting reagent for the next step.