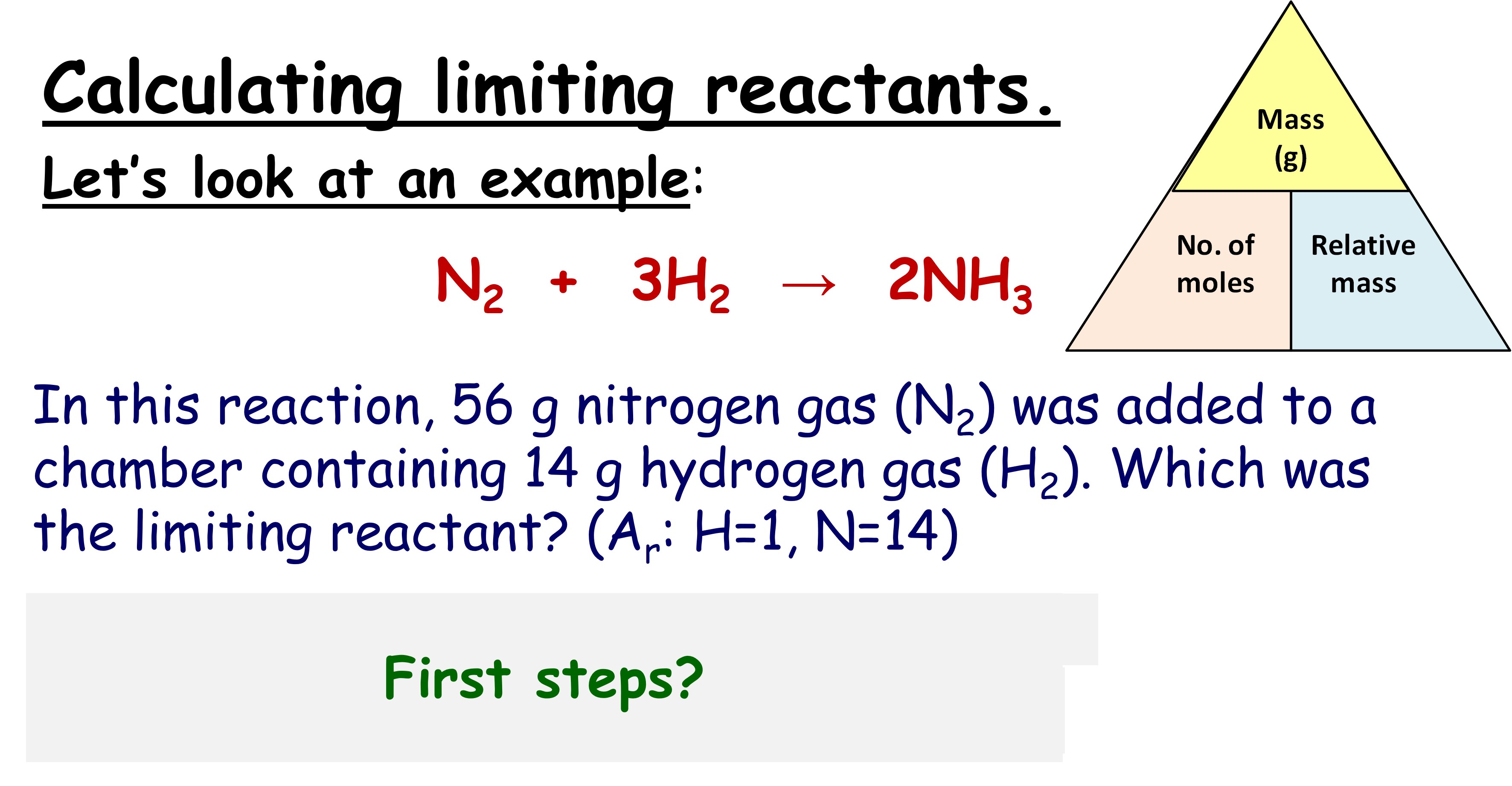
5 Ways to Identify Limiting Reagents Easily

In chemistry, particularly in stoichiometry, understanding how to identify limiting reagents is crucial for students and professionals alike. A limiting reagent (or limiting reactant) is the substance that gets completely consumed in a chemical reaction, thereby limiting the amount of product that can be formed. In this blog post, we will delve into the methods by which one can identify the limiting reagent in a chemical equation with ease, ensuring that your experiments and calculations yield accurate results.
1. Understand the Chemical Equation

Before you can identify a limiting reagent, you need to have a clear understanding of the balanced chemical equation involved in your reaction. This includes knowing the reactants and products, their respective states, and the stoichiometric coefficients.
- Balance the Equation: Ensure that the equation is balanced. This means the number of atoms for each element should be the same on both sides of the reaction arrow.
- Identify Stoichiometry: The coefficients in the balanced equation represent the mole ratio between the reactants and products.
2. Determine the Amounts of Reactants

Knowing how much of each reactant you have is fundamental. Here’s how to proceed:
- Measure in Moles: Convert the given mass or volume of each reactant into moles. This involves dividing by the molar mass or using concentration if in solution.
- Express Ratios: Determine the molar ratio as dictated by the coefficients from the balanced equation.
⚗️ Note: If a reactant is in excess, its quantity doesn't necessarily reflect its involvement in the reaction.
3. Apply Mole Ratios

The next step involves using the mole ratios from the equation to figure out which reactant will run out first:
- Mole Calculation: For each reactant, calculate how many moles of product it can produce based on its initial amount and the stoichiometric ratio.
- Comparison: Compare these amounts. The reactant that produces the least amount of product is the limiting reagent.
4. Use Dimensional Analysis
Dimensional analysis is an effective tool for solving chemical problems:
| Reactant | Moles Given | Mole Ratio | Moles of Product |
|---|---|---|---|
| Reactant A | 2 mol | 1:1 | 2 mol |
| Reactant B | 3 mol | 2:1 | 1.5 mol |
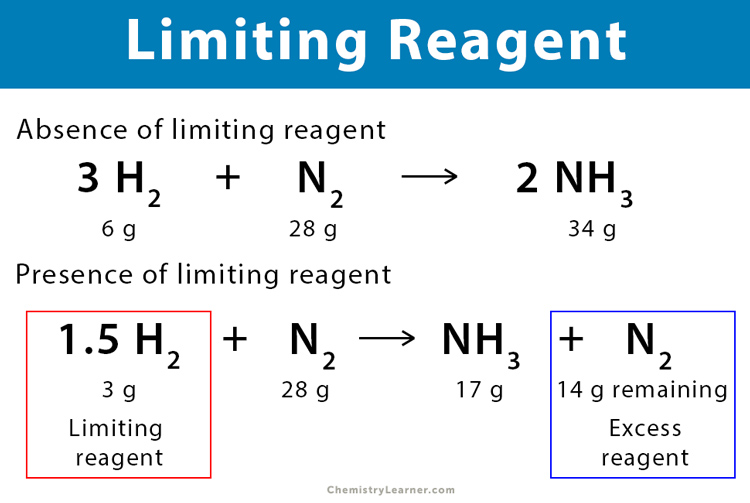
In the example above, Reactant B, producing fewer moles of product, is the limiting reagent.
5. Empirical Observation

Sometimes, an empirical approach can help identify limiting reagents:
- Visual Inspection: If the reaction is observable (e.g., color change or gas formation), you might notice which reactant stops reacting first.
- Experimentation: Perform the reaction with various ratios of reactants to find the "breakpoint" where one reactant becomes the limiting factor.
🔬 Note: Be cautious with this method; reactions might be complex with multiple possible pathways.
By now, you should have a solid foundation in identifying limiting reagents. This knowledge not only helps in academic settings but also in practical applications in industries like pharmaceuticals, where controlling reaction yields is paramount. From balancing equations to using mole ratios and dimensional analysis, these methods ensure you can accurately determine which chemical substance will limit your reaction.
Why do I need to identify a limiting reagent?

+
Identifying the limiting reagent allows you to calculate the theoretical yield of your reaction, predict the amounts of products formed, and manage costs in industrial settings by avoiding unnecessary excess.
What if all reactants are used up at the same time?

+
This is a rare but perfect stoichiometric scenario. Here, there are no excess reactants, and you have what is called a “stoichiometric mixture”.
Can the temperature affect which reactant is limiting?

+
Temperature can indeed change reaction rates, but the limiting reagent itself is determined by the amounts present, not by temperature.
How does identifying the limiting reagent help in waste management?

+
Knowing the limiting reagent helps minimize waste by using just the right amount of each reactant, thus reducing excess materials that would not contribute to the final product.
What if I have more than one reactant in excess?

+
If you have more than one reactant in excess, any one of them could theoretically act as the limiting reagent if the others were in different quantities. The actual limiting reagent would still be the one producing the least product based on the given amounts.