Limiting Reagent Worksheet: Master Your Chemistry Skills Now
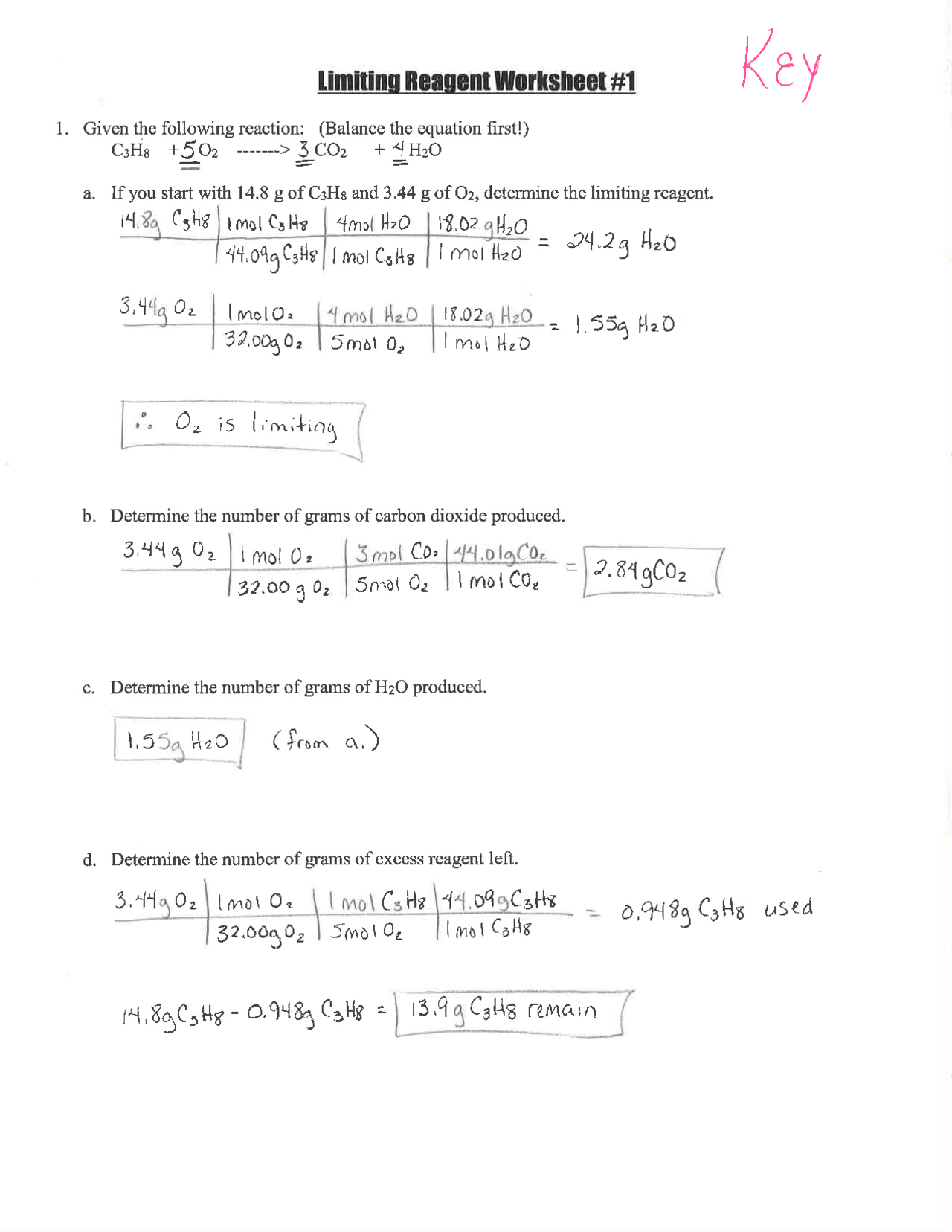
Embarking on the journey of chemistry often brings you face-to-face with a crucial concept known as limiting reagents (or reactants). This fundamental principle is critical for understanding the stoichiometry of chemical reactions. Essentially, the limiting reagent is the reactant that is entirely consumed when a reaction goes to completion, thus determining the quantity of product that can be formed. In this comprehensive guide, we'll dive deep into what limiting reagents are, how to identify them, and most importantly, how to master solving related problems through a worksheet approach.
What is a Limiting Reagent?
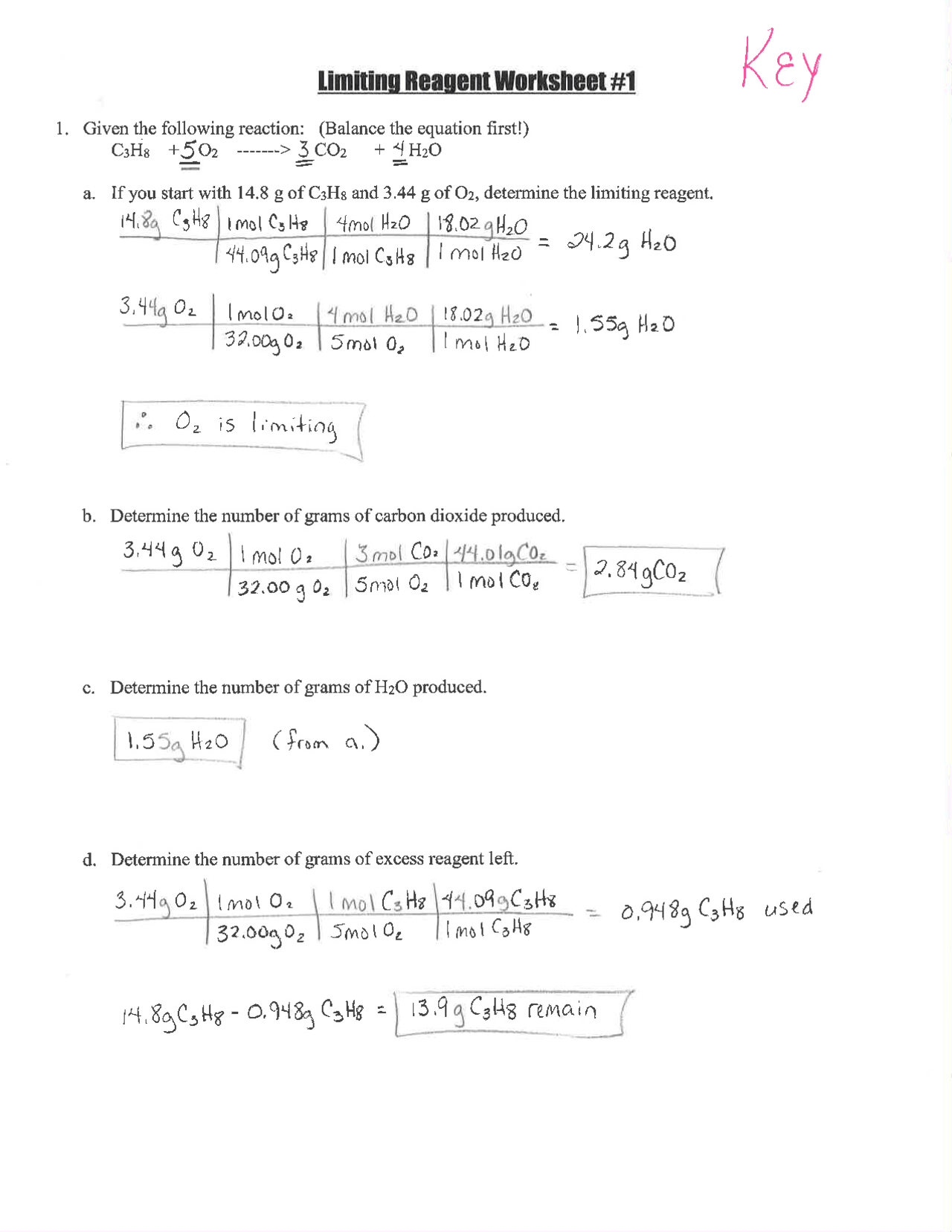
In any chemical reaction, reactants combine in specific ratios to form products. However, in reality, the reagents are seldom available in perfect stoichiometric quantities. When this happens, one of the reactants limits the amount of product that can form. Here’s how you can identify the limiting reagent:
- Calculate the moles of each reactant present.
- Use the balanced chemical equation to determine how many moles of product each reactant would produce if fully consumed.
- The reactant producing the least amount of product is your limiting reagent.
Mastering Limiting Reagents with a Worksheet

To become proficient in identifying limiting reagents, practice is key. Here’s a worksheet-style approach to help you understand and apply the concept:
Step 1: Understand the Chemical Equation
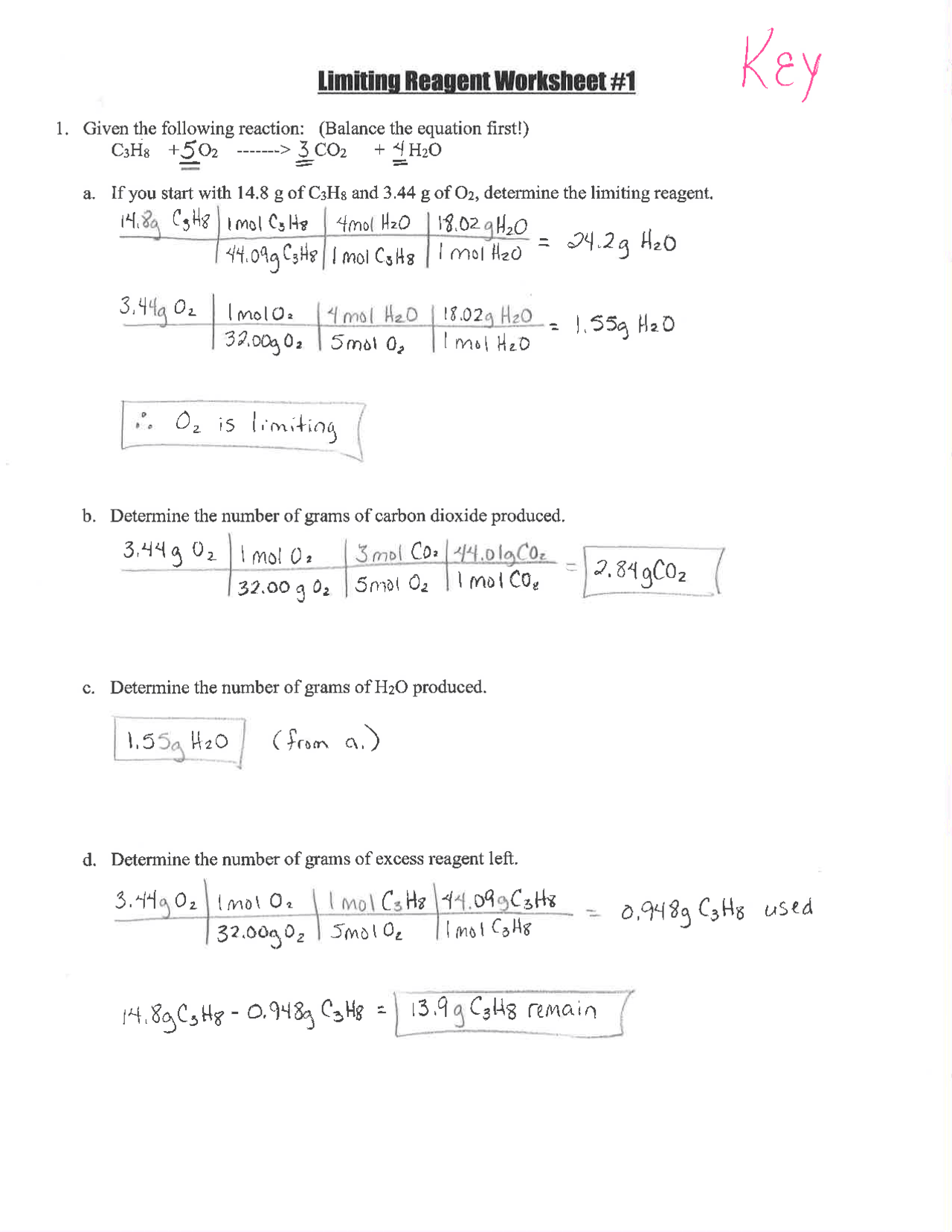
First, ensure you understand the balanced equation. For instance, consider the synthesis of ammonia:
| N2 + 3H2 | → | 2NH3 |
|---|

From this equation, we see that 1 mole of N2 needs 3 moles of H2 to produce 2 moles of NH3.
Step 2: Determine the Moles of Each Reactant
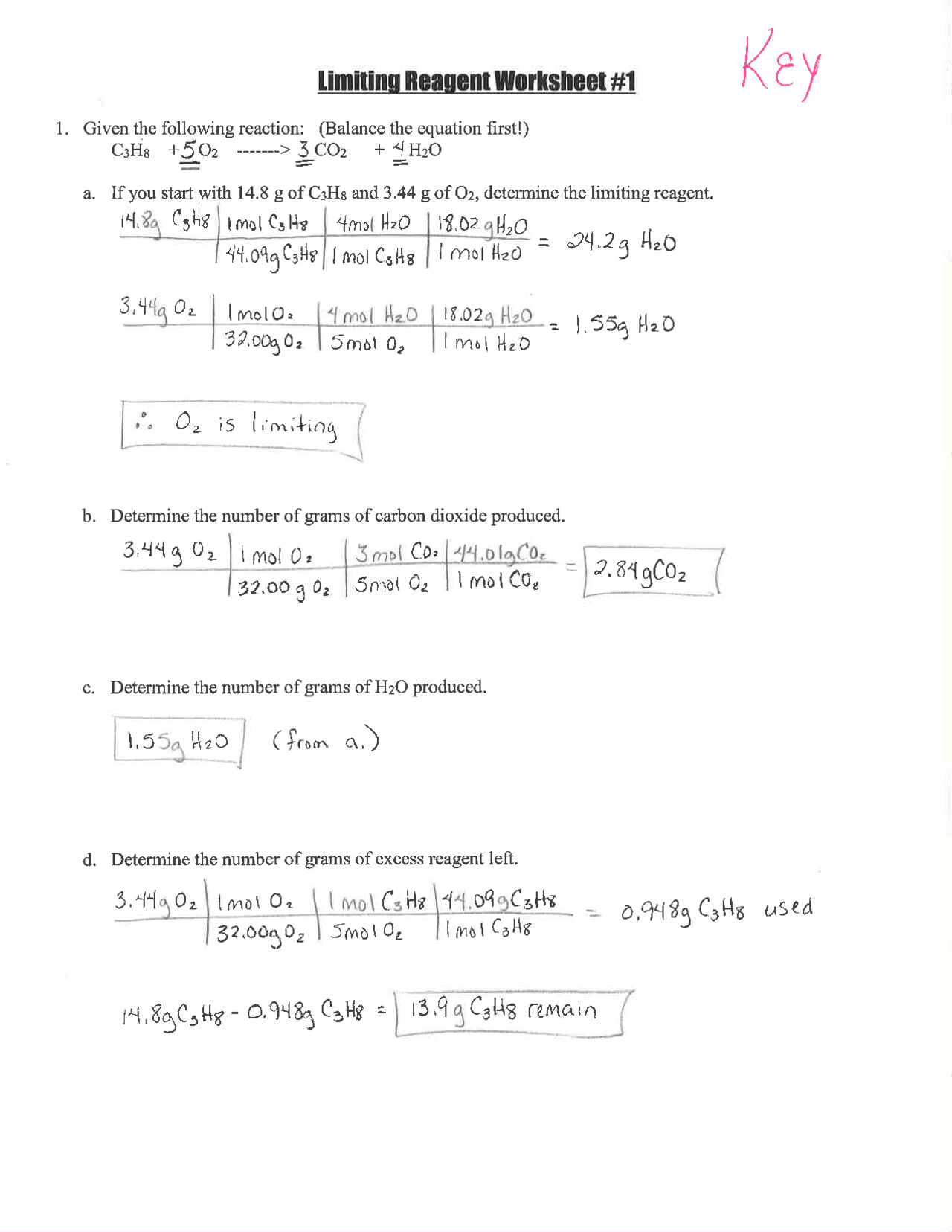
If you have 2 moles of N2 and 5 moles of H2, how much ammonia can be produced?
Step 3: Identify the Limiting Reagent

Calculate:
- If all N2 were consumed, the yield would be:
- 2 moles N2 * (2 moles NH3 / 1 mole N2) = 4 moles of NH3
- If all H2 were consumed, the yield would be:
- 5 moles H2 * (2 moles NH3 / 3 moles H2) ≈ 3.33 moles of NH3
Since H2 produces less NH3, it is the limiting reagent.
Step 4: Calculate the Maximum Yield

Given that H2 is the limiting reagent, we can produce a maximum of 3.33 moles of NH3.
⚗️ Note: Always check your units and use the correct conversion factors when working with volumes and concentrations.
Common Mistakes and How to Avoid Them

Here are some common pitfalls when working with limiting reagents:
- Not converting masses to moles correctly.
- Misreading or ignoring the stoichiometric coefficients in the balanced equation.
- Calculating the yield from the non-limiting reagent instead of the limiting one.
By continually practicing with limiting reagent worksheets, you'll not only sharpen your stoichiometry skills but also avoid these common errors.
📚 Note: Practice with different types of reactions and variations in reactant quantities to broaden your understanding.
Putting Your Skills to the Test

Here’s a sample problem to test your grasp of limiting reagents:
Problem

Given the reaction:
Zn + 2HCl → ZnCl2 + H2
You have 3.0 moles of Zn and 5.0 moles of HCl. What is the limiting reagent, and how many moles of hydrogen gas can be produced?
Solution

- Moles of Zn: 3.0 moles
- Moles of HCl: 5.0 moles
From the equation:
- Zn → 3.0 moles * (1 mole H2 / 1 mole Zn) = 3.0 moles of H2
- HCl → 5.0 moles * (1 mole H2 / 2 moles HCl) = 2.5 moles of H2
HCl limits the reaction to 2.5 moles of hydrogen gas.
In mastering limiting reagent calculations, you not only enhance your understanding of chemical reactions but also gain practical knowledge of how chemicals interact in real-world scenarios. Through diligent practice with worksheets and understanding the theoretical underpinnings, you’ll become adept at predicting outcomes in chemical processes, which is invaluable for various fields including pharmaceuticals, environmental science, and chemical engineering. Remember to practice regularly, apply the steps methodically, and soon, what seemed complex will become second nature.
Why is it important to identify the limiting reagent?

+
Identifying the limiting reagent helps determine the maximum possible yield of the product in a chemical reaction. This is crucial for optimizing reaction conditions, minimizing waste, and understanding economic aspects of chemical processes.
Can a reaction have more than one limiting reagent?

+
In a given reaction, only one reactant can be the limiting reagent at a time because the term “limiting” implies that this reactant dictates the amount of product formed. However, the limiting reagent can change if the ratio of reactants is altered.
What happens to the excess reactants?

+
Excess reactants remain unreacted after the completion of the reaction. They can be recovered or used in subsequent reactions if possible.