5 Essential Steps for Solving Limiting Reagent Problems
Engaging with chemical reactions in the laboratory often involves calculating how much product you can expect from given reactants. This is where limiting reagents become a critical concept. Understanding how to identify and work with limiting reagents is fundamental in chemistry. Let's dive into the five essential steps for solving limiting reagent problems to make this process simpler and more intuitive.
Step 1: Write and Balance the Chemical Equation

Before diving into calculations, ensure you start with a balanced chemical equation. This step is foundational because:
- It clearly shows the stoichiometric relationship between reactants and products.
- It gives you the proportions in which substances react.
For example, let's consider the reaction between sodium and chlorine:
Step 2: Calculate Moles of Each Reactant
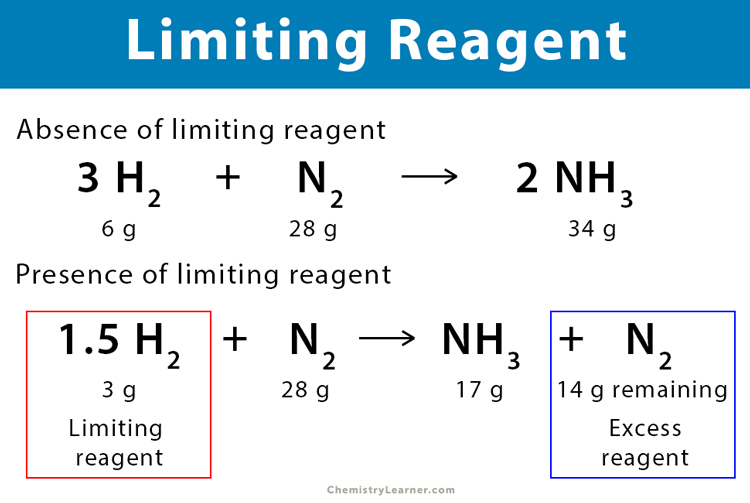
Next, convert the given masses or volumes of reactants into moles using their molar mass or standard conditions for gases:
- If given mass: moles = mass ÷ molar mass
- If given volume at STP for gases: moles = (volume in liters) ÷ 22.4
Assume you have 10 grams of sodium and 10 liters of chlorine gas at STP:
- For sodium, moles = 10g ÷ 22.99g/mol = 0.435 moles
- For chlorine, moles = 10L ÷ 22.4L/mol = 0.446 moles
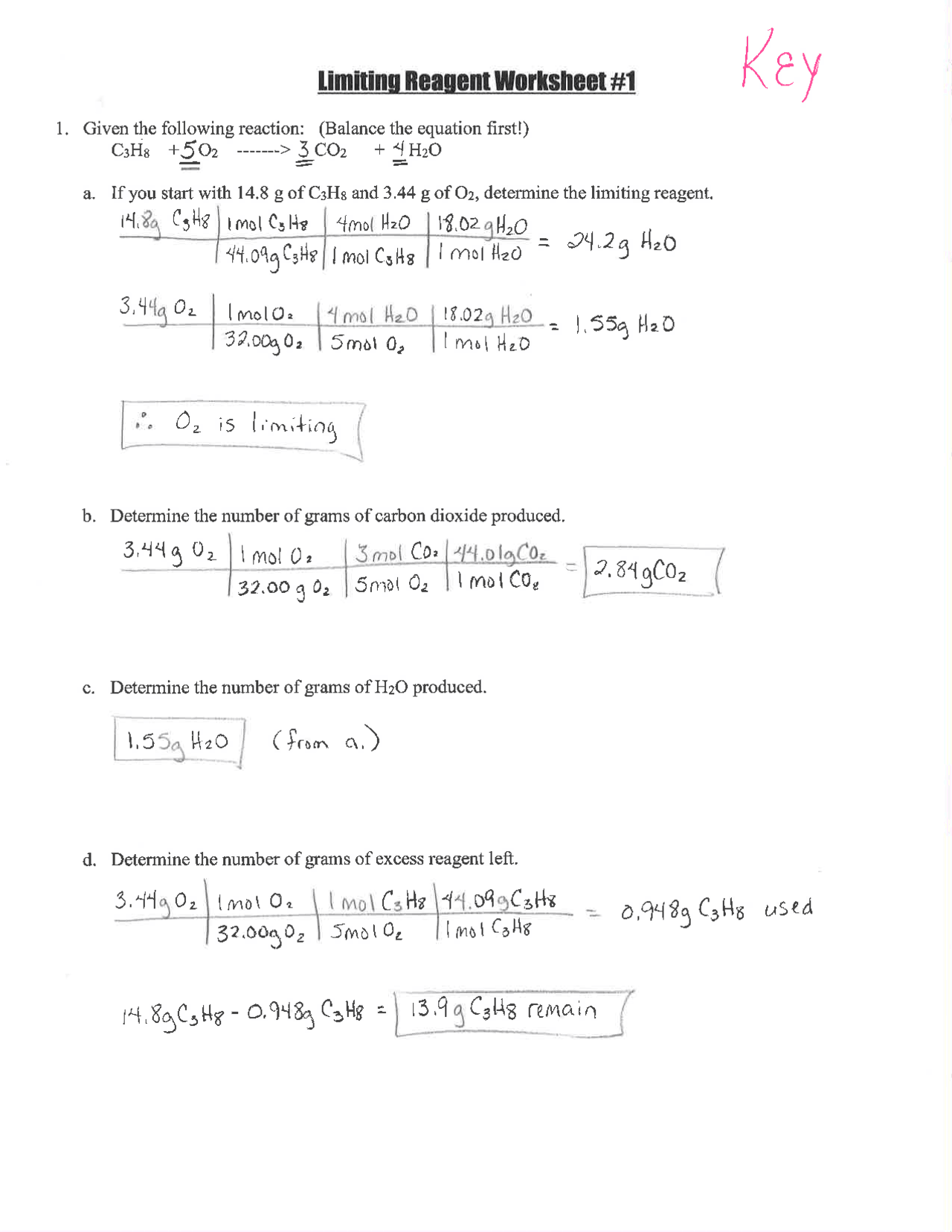
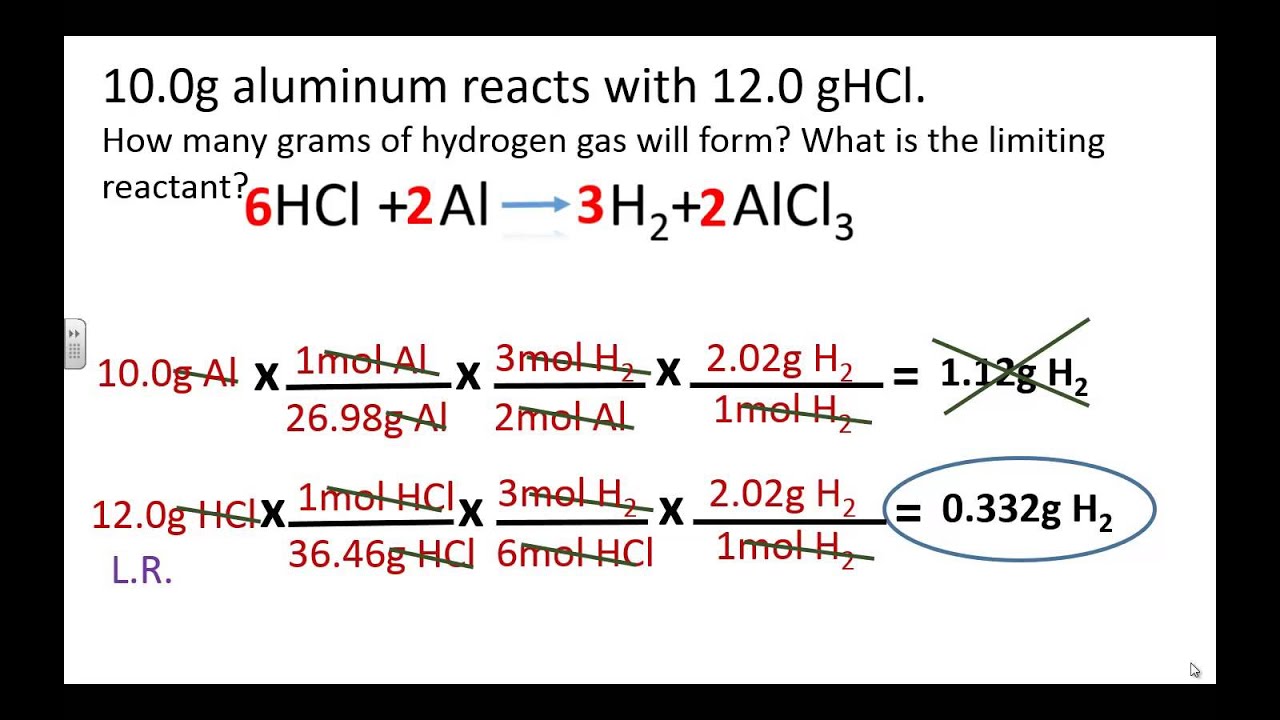
Step 3: Identify the Limiting Reagent Using Mole Ratios

Use the mole ratio from the balanced equation to determine which reactant is in excess:
- The stoichiometric ratio from the equation (Na:Cl2) is 2:1
- Calculate how much chlorine would react with all the sodium: 0.435 moles Na x 1 mole Cl2/2 moles Na = 0.2175 moles of Cl2
- Since you have 0.446 moles of Cl2, it is in excess, making sodium the limiting reagent.
🔍 Note: If the mole ratios don't give you a clear answer, you can calculate how much product each reactant would produce to confirm the limiting reagent.
Step 4: Calculate the Amount of Product Formed
Using the moles of the limiting reagent, you can now calculate the theoretical yield of the product:
- Using the balanced equation, 2 moles of Na give 2 moles of NaCl.
- Thus, 0.435 moles of Na would yield 0.435 moles of NaCl.
To convert this into mass:
- Molar mass of NaCl = 23g/mol + 35.5g/mol = 58.5g/mol
- Yield = 0.435 moles x 58.5g/mol = 25.46g of NaCl
Step 5: Consider Yield and Efficiency

While the calculations give you the theoretical maximum yield, real-world reactions often have less than 100% efficiency due to:
- Purification steps losing some product
- Incomplete reactions
- Side reactions consuming reactants
If given, account for the percent yield:
- Actual yield = Theoretical yield x (% yield / 100)
💡 Note: Always check if the reaction conditions or the percent yield are known to adjust the calculations accordingly.
By following these five steps, you can effectively solve limiting reagent problems, ensuring accurate predictions of reaction outcomes. Whether you're optimizing chemical processes or simply completing a lab experiment, understanding limiting reagents empowers you to use reactants more efficiently and achieve the desired results with precision.
In summary, always balance your equations, convert given amounts to moles, use mole ratios to identify the limiting reagent, calculate the product yield based on the limiting reagent, and factor in any given efficiency or yield data. This approach ensures comprehensive understanding and better lab results.
What if both reactants are present in stoichiometrically equivalent amounts?

+
If the reactants are present in exactly the same stoichiometric ratio as the balanced equation, there will be no limiting reagent; both will be fully consumed. The yield will be based on the given amounts of reactants without any excess.
How can I determine the percent yield of a reaction?
+
Percent yield is calculated by dividing the actual yield (what you obtained in the lab) by the theoretical yield (what you should have obtained according to calculations) and then multiplying by 100. The formula is: (Actual Yield / Theoretical Yield) x 100.
Can you explain why my calculated yield is lower than expected?

+
Your yield might be lower due to various reasons like incomplete reactions, side reactions forming by-products, losses during isolation or purification steps, and human or equipment error. Ensure all these factors are considered in your experiment or calculation adjustment.