5 Essential Steps to Master Limiting Reactants

In the realm of chemistry, understanding limiting reactants is fundamental for mastering stoichiometry and reaction analysis. A limiting reactant is the substance that gets used up first in a chemical reaction, thereby determining the maximum amount of product that can be formed. Here's how you can become proficient in identifying and working with limiting reactants through these essential steps:
Step 1: Write the Balanced Chemical Equation

The foundation of calculating limiting reactants begins with the balanced chemical equation. This equation shows the reactants and products along with their stoichiometric coefficients, which are essential for determining the ratio in which substances react.
- Identify all reactants and products.
- Balance the equation to ensure the conservation of mass.
Step 2: Calculate the Moles of Each Reactant
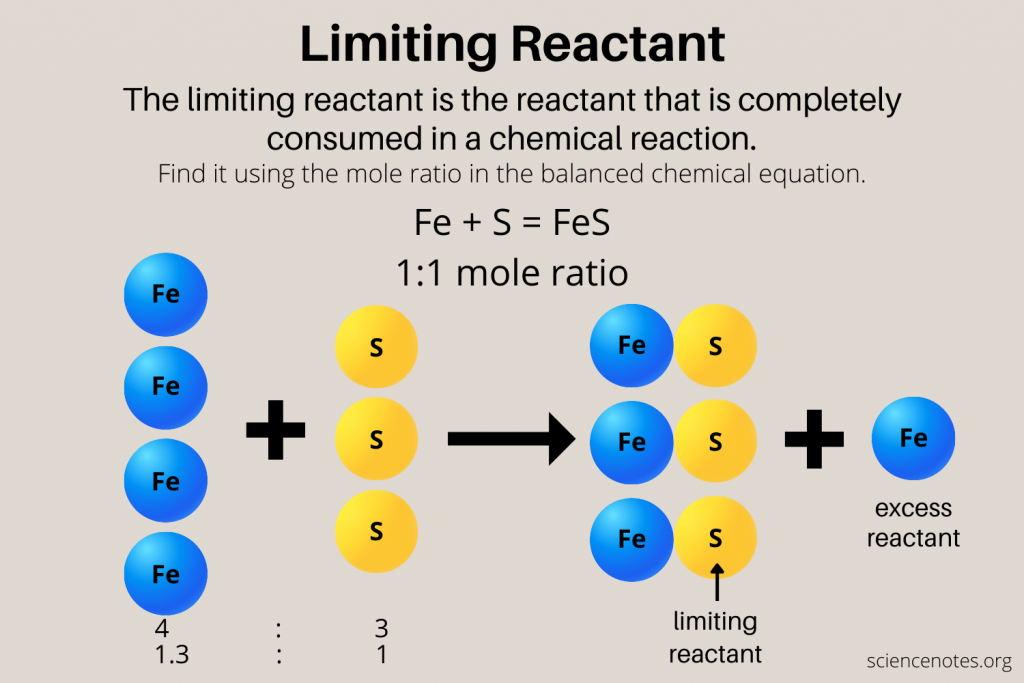
To find out which reactant is limiting, first calculate the moles of each reactant you have:
- Use the formula: moles = mass / molar mass.
- Convert given masses or volumes to moles using the molar mass or molar volume (in case of gases).
🔎 Note: Ensure you're using the correct molar mass for each reactant. Mistakes here will throw off your calculations.
Step 3: Use the Balanced Equation to Determine Mole Ratios

With the moles calculated, compare these amounts to the stoichiometric coefficients in the balanced equation to find out which reactant will run out first:
- Divide the moles of each reactant by its coefficient in the balanced equation.
- The reactant with the smallest result after division will be the limiting reactant.
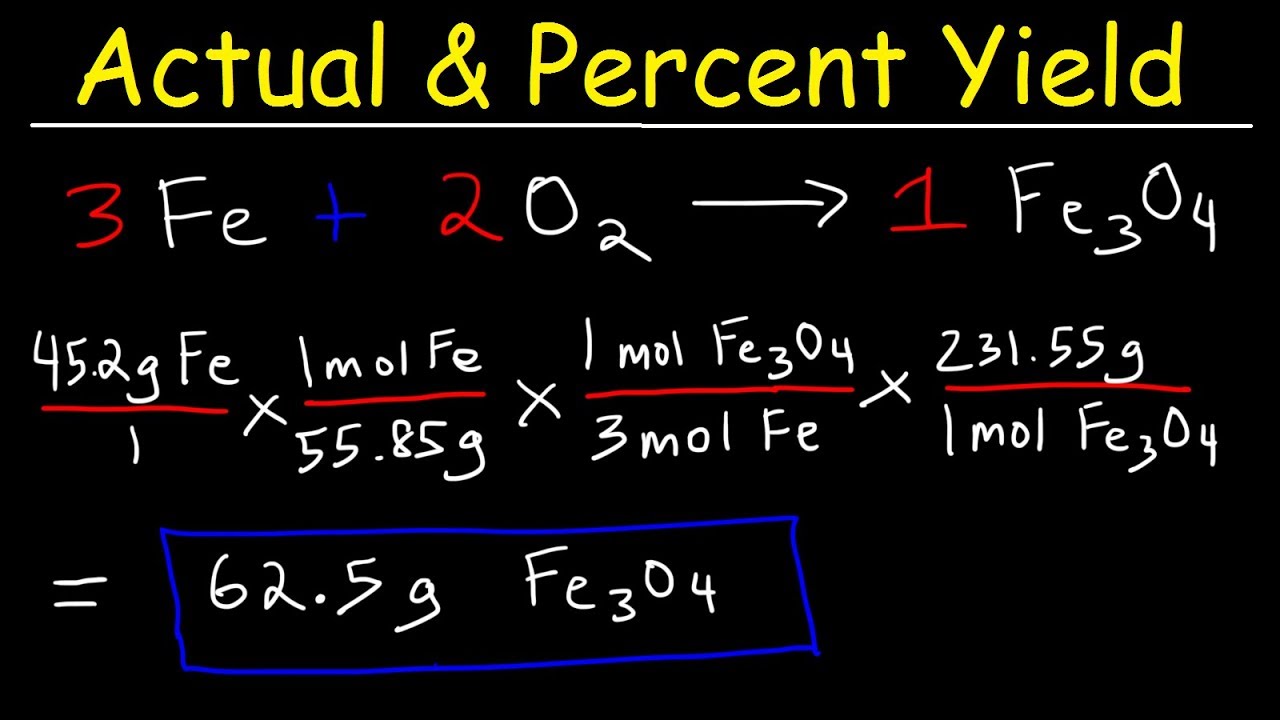
Step 4: Calculate the Yield Based on the Limiting Reactant

Now that you know which reactant is limiting, you can calculate the theoretical yield of the product:
- Multiply the moles of the limiting reactant by the mole ratio between the product and the limiting reactant from the balanced equation.
- Convert moles of the product to mass using its molar mass to get the theoretical yield.
Step 5: Understand the Implications

Knowing the limiting reactant has several practical implications:
- Product Quantities: The amount of product formed is directly dependent on the amount of limiting reactant available.
- Excess Reactants: Other reactants will be in excess, meaning they will not be entirely used up in the reaction.
- Efficiency of Reactions: Understanding the limiting reactant helps in optimizing reactions to avoid waste.
📝 Note: Always be aware that real reactions often have side reactions or impurities, which can affect the yield and the determination of the limiting reactant.
By following these steps, you'll not only grasp the concept of limiting reactants but also enhance your ability to predict and quantify reaction outcomes. Mastering this skill is crucial for both academic and industrial applications of chemistry, from pharmaceutical synthesis to environmental analysis.
This proficiency allows you to:
- Predict the maximum amount of product that can be made.
- Calculate how much of an excess reactant will remain after the reaction.
- Optimize the conditions of the reaction to increase yield or reduce cost.
💡 Note: Practice with different reactions and scenarios is key to solidifying your understanding of limiting reactants. Work through exercises, study case studies, and consider real-world applications to deepen your insight.
Embarking on this journey to master limiting reactants equips you with not just the technical skills but also with the problem-solving mindset necessary for advanced studies in chemistry. This knowledge is indispensable for designing experiments, planning syntheses, and understanding the outcomes of chemical reactions on a microscopic scale. Let your mastery of limiting reactants propel you towards greater achievements in the field of chemistry.
What if there is no limiting reactant?

+
If there is no limiting reactant, it means all reactants are used up simultaneously, which is rare in real-world scenarios due to the difficulty in achieving perfect stoichiometric ratios. Typically, at least one reactant will be in excess.
How do you handle impurities or side reactions when calculating yields?

+
Impurities or side reactions can be accounted for by using percent yield calculations, which compare the actual yield to the theoretical yield. Adjustments might also be made to consider known side products or incomplete reactions.
Can the limiting reactant change during the reaction?

+
In typical scenarios, once the reaction starts, the limiting reactant does not change. However, if new reactants are added or if the reaction conditions change significantly, the limiting reactant might shift.
What is the significance of identifying the limiting reactant?
+
Identifying the limiting reactant is crucial because it:
- Determines the theoretical yield of the reaction.
- Helps in understanding which reactant to add more or less of in order to optimize the reaction.
- Allows for better planning and resource allocation in industrial processes.
How can I practice identifying limiting reactants?

+
Practice can be enhanced by:
- Working through textbook problems.
- Simulating chemical reactions online or with software.
- Conducting actual lab experiments where you measure reactants and products.