5 Easy Ways to Master Limiting Reactant Problems

Ever grappled with the challenge of limiting reactant problems in chemistry? You're not alone. These problems are a cornerstone of stoichiometry, and mastering them is key to excelling in both academic and practical chemistry applications. Here are five straightforward methods to tackle these problems with confidence:
1. Identify the Limiting Reactant
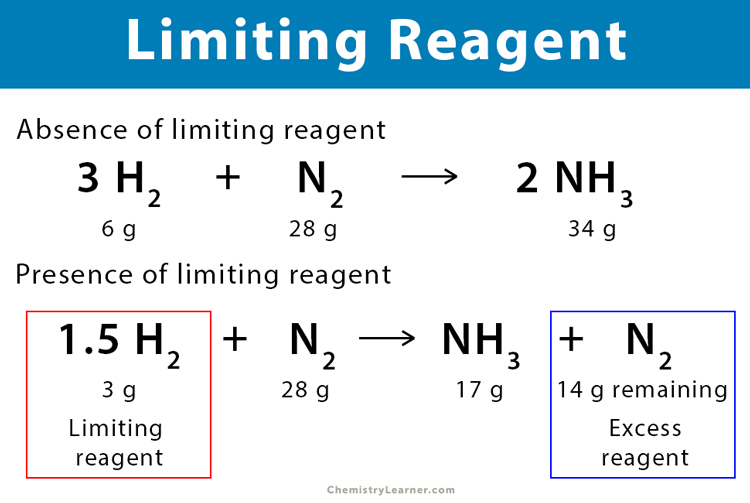
The first step in mastering limiting reactant problems is to identify which reactant limits the reaction. Here’s how:
- Balance the Chemical Equation: Ensure the chemical equation is balanced to understand the mole ratios between reactants and products.
- Convert Given Amounts to Moles: Convert the masses or volumes of reactants to moles using their respective molecular weights or concentrations.
- Calculate the Mole Ratio: Use the balanced equation to calculate how many moles of each reactant are needed and available.
- Compare Moles: Determine which reactant will be used up first by comparing the moles needed versus the moles available.
🧑🏫 Note: The reactant producing the least amount of product based on the balanced equation is your limiting reactant.
2. Use Stoichiometry to Calculate the Yield

Once you’ve found the limiting reactant, use it to calculate how much product can be formed:
- Take the amount of limiting reactant in moles.
- Apply the mole ratio from the balanced equation to find moles of product.
- Convert these moles to grams (or the unit required) using the molar mass of the product.
This method ensures you calculate the theoretical yield accurately, which is critical for understanding the efficiency of chemical reactions.
3. Understand the Concept of Excess Reactant

Every reaction leaves some reactants behind:
- Calculate the amount of excess reactant by subtracting the moles used in the reaction from the moles initially present.
- Convert this remaining amount back to mass or volume to determine how much is left unreacted.
This understanding helps in optimizing chemical processes, reducing waste, and managing inventory in industrial settings.
4. Practice with Real-World Scenarios

Theory can only take you so far; practical application is where mastery truly begins:
- Engage in lab experiments where you can physically measure and calculate limiting reactants.
- Work through chemical equations related to real-life applications like food processing, manufacturing, or environmental analysis.
🔬 Note: Real-world application often involves variables like purity, yield percentages, or unexpected reactions, providing a deeper understanding of stoichiometry.
5. Leverage Digital Tools

Technology can be a fantastic aid in learning:
- Simulations and Virtual Labs: Use online chemical simulations to experiment with different amounts of reactants and observe the outcomes.
- Calculators and Apps: Stoichiometry apps can help automate the tedious calculations, allowing for faster practice and reinforcement of the concepts.
By integrating digital tools, you can accelerate your learning curve and deepen your understanding of how chemicals interact.
To sum it up, mastering limiting reactant problems involves recognizing the reactant that runs out first, calculating product yields, understanding excess reactants, applying these concepts in practical scenarios, and leveraging technology for efficiency. Each method builds upon the last, creating a solid foundation in chemical calculations, which is invaluable in academic pursuits or in careers within the chemical industry.
Why do we need to identify the limiting reactant in a reaction?

+
The limiting reactant is the substance that is completely used up first during a chemical reaction, determining how much product can be formed. This calculation helps in predicting yield, managing resources, and optimizing the cost and efficiency of chemical processes.
What happens if you don’t identify the limiting reactant?

+
Without knowing the limiting reactant, you might end up overestimating the amount of product you can produce, leading to wasted resources, increased costs, and potentially incomplete reactions.
Can technology replace the manual calculation of limiting reactants?
+
While technology can automate calculations, understanding the underlying principles is crucial. Technology should be used as a tool to enhance learning, not to replace it, as manual practice reinforces the conceptual understanding.
How can I practice stoichiometry at home?

+
You can use online platforms for virtual labs, stoichiometry apps, or engage in DIY home experiments where you can measure out reactants and observe the reactions. It’s also beneficial to practice with textbook problems or use educational websites that provide step-by-step solutions.
What are the common mistakes to avoid when solving limiting reactant problems?

+
Common mistakes include not balancing the chemical equation, miscalculating the mole ratios, failing to convert all measurements to moles, and overlooking the concept of excess reactant. Ensure you understand and apply each step methodically.