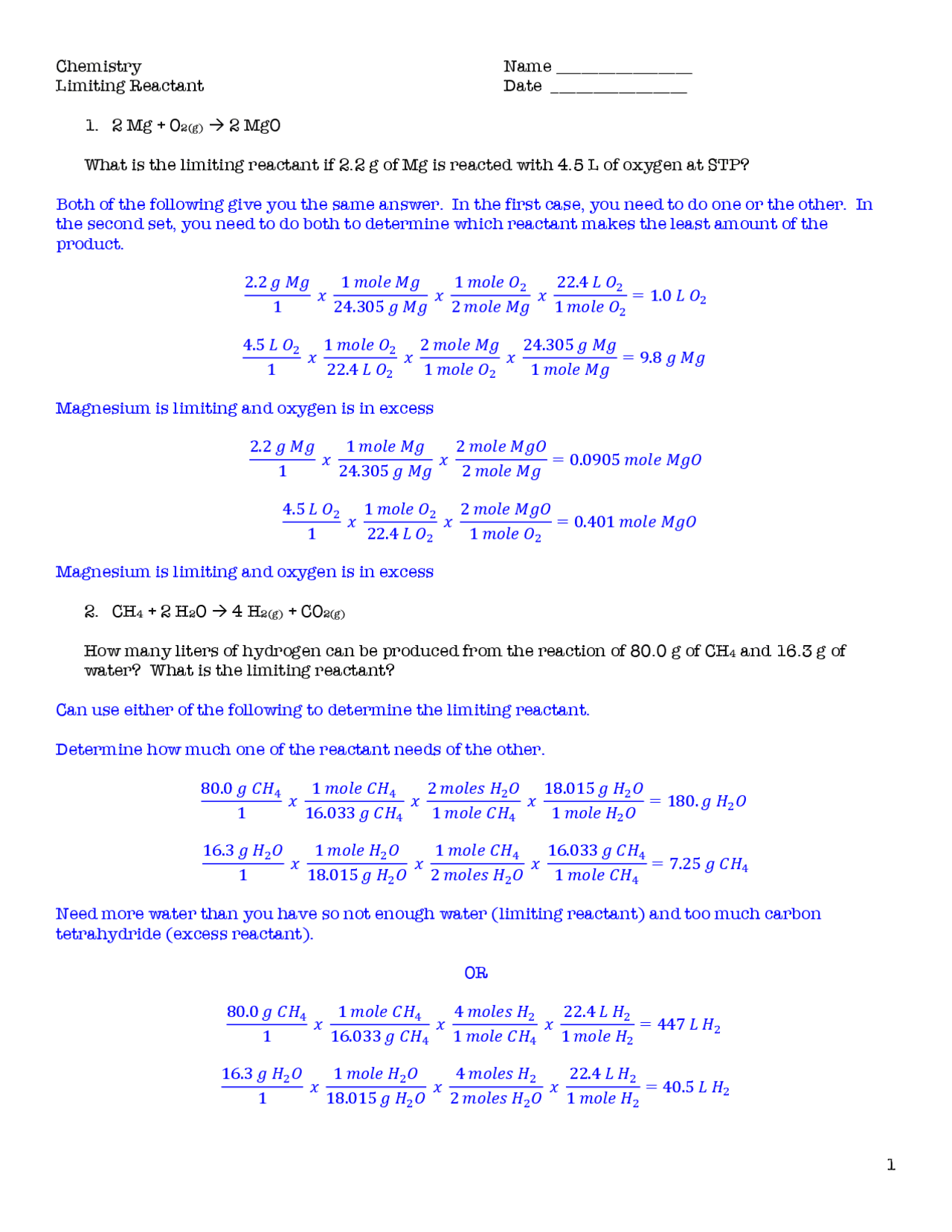
5 Tips for Solving Limiting Reactant and Percent Yield Problems

In the realm of chemistry, two of the most challenging types of calculations often deal with the concepts of limiting reactant and percent yield. These calculations not only require a firm understanding of stoichiometry but also demand precision and a systematic approach. Whether you're a high school student grappling with AP Chemistry or a college student delving into more complex chemical reactions, mastering these calculations can be a game changer for your understanding and performance in chemistry. Here are five tips to help you solve limiting reactant and percent yield problems effectively:
1. Identify the Limiting Reactant
The first step in any limiting reactant problem is to determine which reactant will be used up first, thereby limiting the amount of product that can be formed. Here's how you can do it:
- Convert all reactant quantities to moles using their molar masses.
- Use the balanced chemical equation to find the mole ratio of each reactant to the product. For example, if the equation is 2A + B → AB₂, the ratio of A to B is 2:1.
- Calculate how many moles of product each reactant would produce based on their moles and the stoichiometry ratio.
- The reactant that produces the least amount of product is your limiting reactant.
2. Calculate the Theoretical Yield

Once you've identified the limiting reactant, use its quantity to find out how much product should theoretically be produced:
- From the limiting reactant, determine the amount of product it can form.
- This amount is your theoretical yield.
| Reactant | Amount (mol) | Product Yield (mol) |
|---|---|---|
| Limiting Reactant A | X mol | Y mol (theoretical yield) |

3. Find the Percent Yield

Percent yield reflects the efficiency of a chemical reaction, comparing the actual yield (what you get in the lab) to the theoretical yield:
Percent Yield = (Actual Yield / Theoretical Yield) × 100%
- Calculate the actual yield from your experimental results.
- Plug both the actual and theoretical yields into the formula to find the percent yield.
🔗 Note: Remember that the actual yield will never exceed the theoretical yield due to unavoidable losses or inefficiencies in reactions.
4. Use Dimensional Analysis for Conversions

Dimensional analysis (or the factor-label method) is your best friend when it comes to stoichiometric calculations. Here's how to utilize it:
- Convert the given amount of any reactant or product into moles.
- Use mole ratios from the balanced equation to convert from one substance to another.
- Ensure you check units cancel out correctly to avoid common mistakes.
5. Practice and Visualize the Problem

Mastery comes with practice, and visualizing the problem can often clarify complex steps:
- Try using particle diagrams to see how reactants transform into products.
- Set up practice problems where you know the limiting reactant and have to backtrack to find the initial quantities or percent yield.
- Work through examples from textbooks or online resources, focusing on understanding each step.
Understanding limiting reactant and percent yield problems is essential for not only passing chemistry exams but also for grasping the practical aspects of chemical synthesis. These calculations illustrate how much of a chemical product can be produced from given reactants, providing insights into reaction efficiencies and reagent usage. Here's a quick recap:
- Identify the limiting reactant by comparing the moles of product each reactant can produce.
- The theoretical yield is the maximum amount of product you can expect from the limiting reactant.
- Calculate percent yield by comparing actual yield with the theoretical yield.
- Employ dimensional analysis to keep your units straight during conversions.
- Practice regularly and use visual aids to better understand the relationships between reactants and products.
By following these five tips, you can systematically approach and solve problems involving limiting reactants and percent yield, enhancing both your learning experience and your proficiency in chemistry.
Why is it important to identify the limiting reactant?
+
Identifying the limiting reactant is crucial because it determines how much product can be formed in a chemical reaction. Without knowing the limiting reactant, you might miscalculate the theoretical yield and subsequently, the percent yield.
Can percent yield ever be greater than 100%?

+
No, percent yield cannot exceed 100%. If it does, there might be an error in calculation or contamination of the product with impurities, increasing the apparent yield.
What’s the significance of the molar mass in these calculations?

+
Molar mass converts the mass of reactants and products to moles, which is essential for stoichiometric calculations. Without this conversion, you wouldn’t be able to relate the quantities of substances in the reaction to their actual yield or the limiting reactant.
How does using excess reactants affect the reaction?

+
Using excess reactants ensures that the limiting reactant is completely consumed, maximizing the amount of product formed. It also helps in driving the reaction forward, especially if it is reversible, by Le Chatelier’s principle.