5 Simple Tips for Limiting and Excess Reactants Worksheets

Balancing chemical equations is a fundamental skill in chemistry that ensures the conservation of mass and energy in reactions. One crucial aspect of this process is understanding the concept of limiting and excess reactants. This understanding not only helps in predicting the outcomes of chemical reactions but also in real-world applications, from pharmaceutical production to environmental protection. Here, we explore five simple tips to master worksheets involving limiting and excess reactants.
Understand the Concept
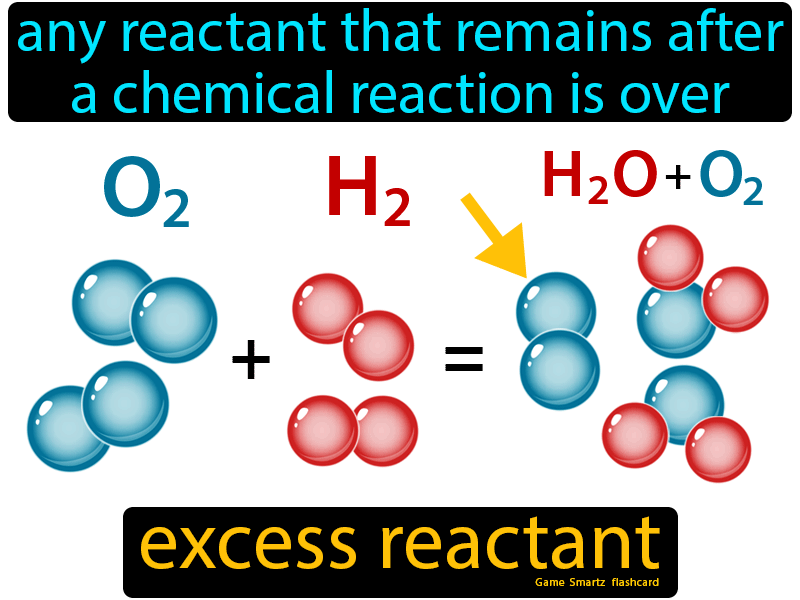
What are Limiting and Excess Reactants?
In any chemical reaction, reactants are not always used up at the same rate. Typically, one reactant is completely consumed before the others, dictating how much product can be made. This reactant is known as the limiting reactant. Conversely, any remaining reactants after the reaction are called excess reactants. To grasp this:
- Start with a basic definition: limiting reactant - the reactant that gets completely used up in a chemical reaction.
- Recognize that the amount of product formed is dictated by this limiting reactant.
Set Up Your Worksheet

Preparing your worksheet properly can make the process of identifying limiting and excess reactants much smoother:
- Use a stoichiometry table or ice chart (Initial, Change, Equilibrium) to organize your information.
- List the reactants with their given amounts at the top of the worksheet.
- Calculate the moles or masses needed for each reactant to react completely, based on the balanced equation.
Calculate the Theoretical Yields

To determine which reactant is limiting:
- Compute the amount of product each reactant would produce if it reacted fully with the other reactants. This is known as theoretical yield.
- Identify the reactant that yields the smallest amount of product; this is your limiting reactant.
| Reactant | Given Amount | Theoretical Yield |
|---|---|---|
| Reactant A | 5 moles | 10 moles of product |
| Reactant B | 3 moles | 6 moles of product |

Practice Stoichiometry
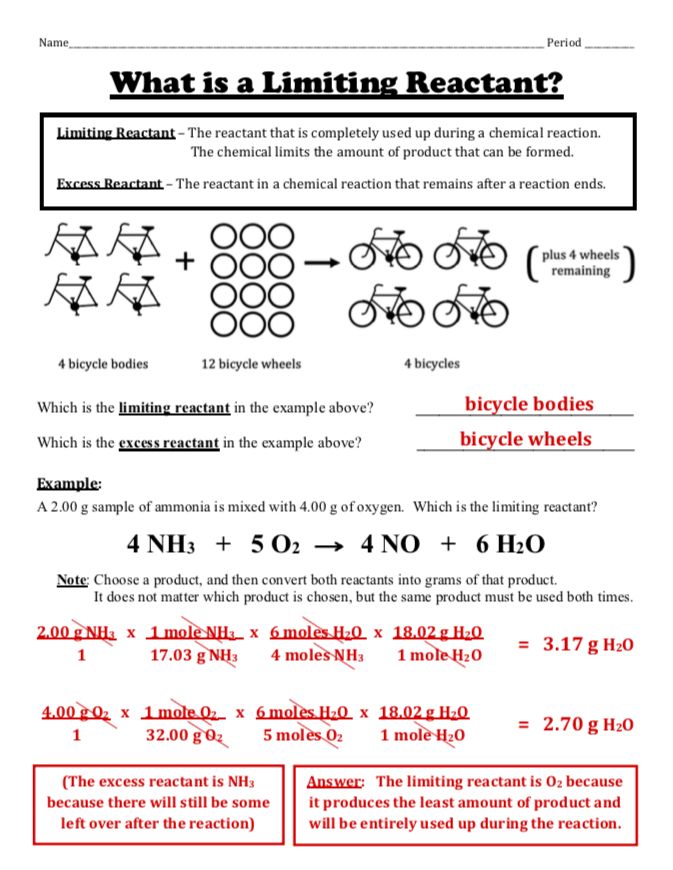
Practice is key to mastering the calculation of limiting and excess reactants:
- Work through numerous examples of stoichiometry problems focusing on limiting reactant scenarios.
- Start with simpler reactions involving just two reactants, then progress to more complex systems.
💡 Note: Make sure to balance the chemical equation before performing any calculations to avoid errors.
Analyze and Learn

After solving several problems:
- Look for patterns or common methods used in solving these types of problems.
- Analyze your mistakes to understand where common errors occur.
- Create a small reference card or a mind map to remember key steps or formulas.
Understanding limiting and excess reactants through worksheets is not just about crunching numbers; it's about grasping the dynamics of chemical reactions. By using these tips, you can enhance your ability to predict reaction outcomes, optimize processes in industrial settings, or simply pass your next chemistry exam. Remember, every time you work on these problems, you're not only solving for reactants but also improving your problem-solving skills, which are valuable in various scientific and real-life scenarios.
How can I tell which reactant is limiting?

+
To determine the limiting reactant, calculate the theoretical yield for each reactant. The reactant that produces the least amount of product when fully reacted is the limiting reactant.
Can there be more than one limiting reactant?

+
No, there can only be one limiting reactant in a chemical reaction. However, in a set of reactions, different reactants might be limiting in different steps or scenarios.
Why is understanding excess reactants important?

+
Excess reactants provide insights into the efficiency of a reaction, potential for side reactions, and how to optimize the reaction for better yield or cost-effectiveness.