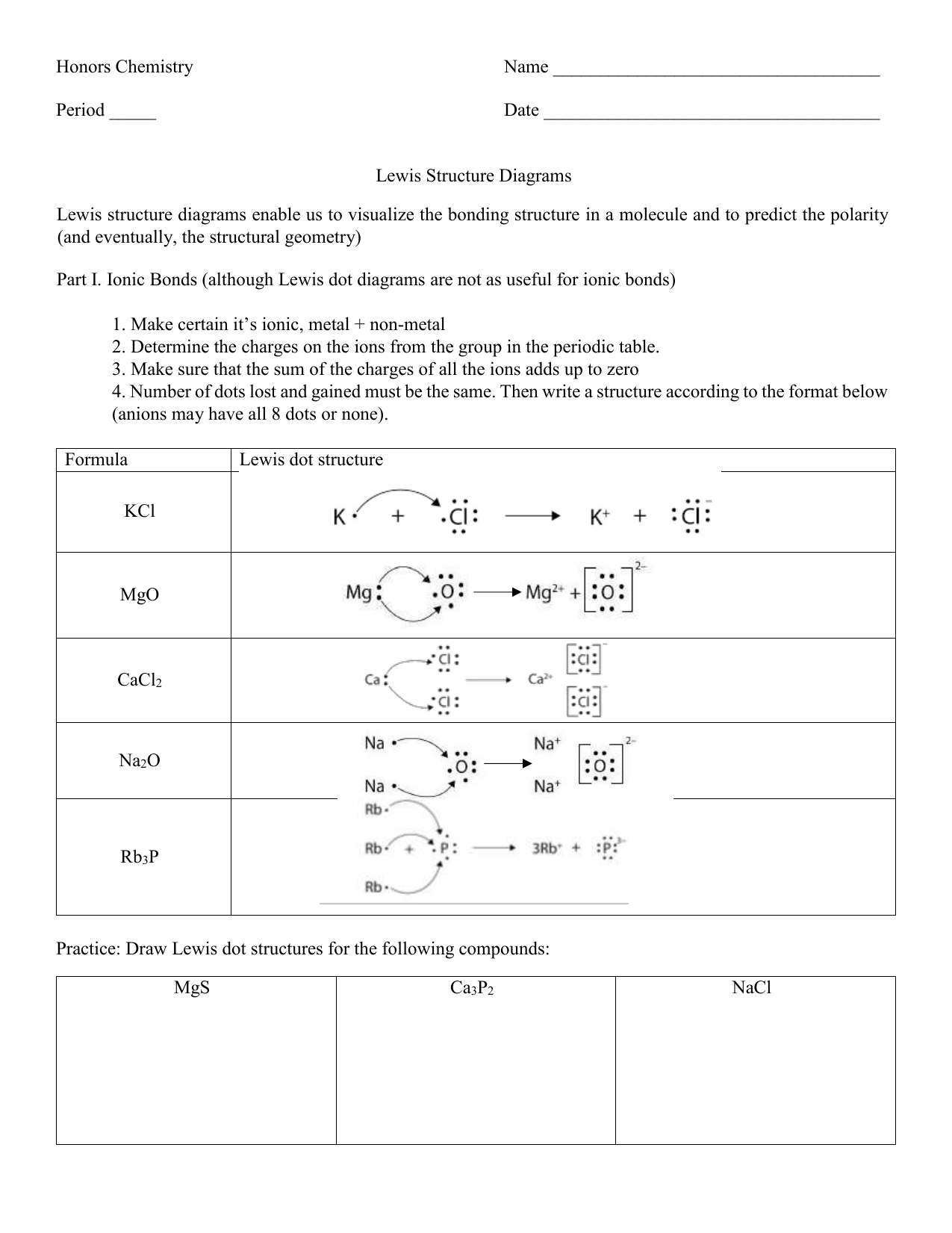
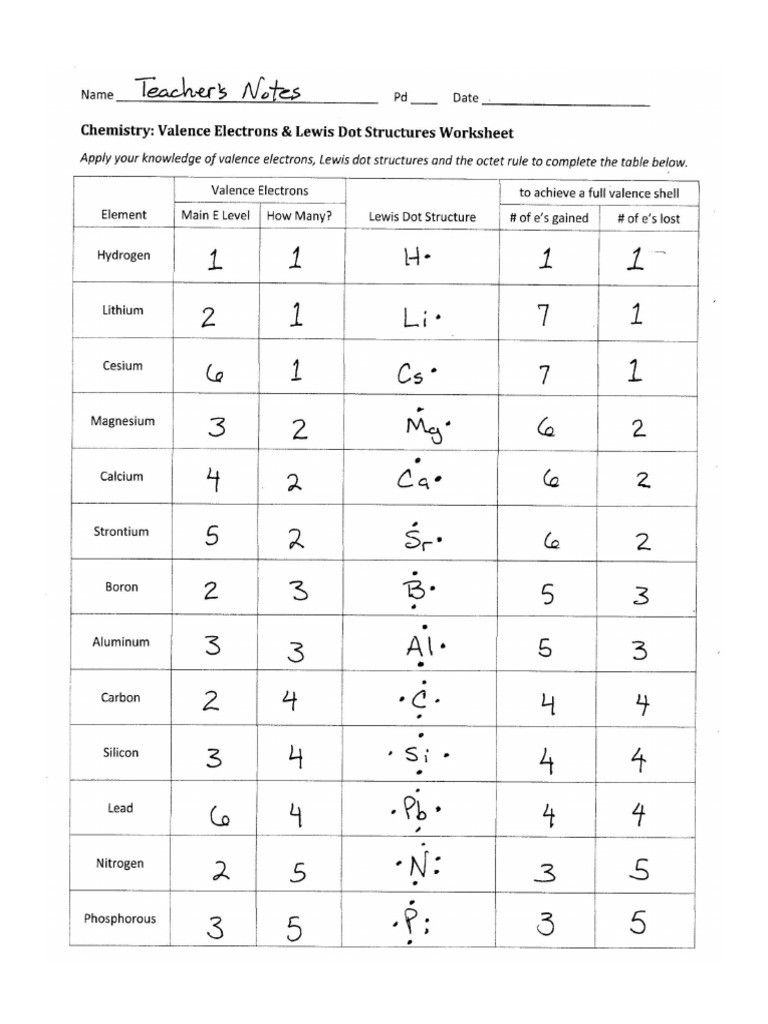
Lewis Structures of Atoms Worksheet

Introduction to Lewis Structures
Lewis structures are a fundamental concept in chemistry, used to represent the bonding between atoms in a molecule. They are a visual representation of the molecular structure, showing how electrons are arranged and shared between atoms. In this article, we will delve into the world of Lewis structures, exploring their importance, how to draw them, and providing a comprehensive worksheet to practice your skills.
What are Lewis Structures?

Lewis structures, also known as electron dot diagrams, are a way of representing the valence electrons of an atom. They are used to show the arrangement of electrons in a molecule, including the bonds between atoms and the lone pairs of electrons. The Lewis structure is a powerful tool for predicting the properties and behavior of molecules.
Why are Lewis Structures Important?

Lewis structures are essential in chemistry because they help us understand the molecular structure and properties of a compound. They are used to: * Predict the shape and polarity of a molecule * Determine the type of bonds between atoms (single, double, or triple) * Identify the lone pairs of electrons and their role in the molecule * Understand the reactivity of a molecule
How to Draw Lewis Structures

Drawing Lewis structures involves several steps: * Determine the total number of valence electrons in the molecule * Draw the symbol of each atom in the molecule * Connect the atoms with single bonds * Distribute the remaining electrons as lone pairs or multiple bonds * Check that each atom has a full outer energy level (except for hydrogen, which only needs two electrons)
Examples of Lewis Structures
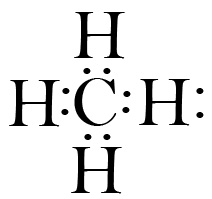
Here are a few examples of Lewis structures: * Water (H2O):
 * Methane (CH4):
* Methane (CH4):  * Ammonia (NH3):
* Ammonia (NH3): 
Lewis Structures Worksheet

Now it’s time to practice drawing Lewis structures! Here are 10 molecules for you to practice: * CO2 * CH3OH * C6H12O6 * NH3 * H2O2 * C2H4 * CH3CH3 * HCN * CH3NH2 * C2H2
📝 Note: When drawing Lewis structures, make sure to follow the octet rule, which states that each atom should have a full outer energy level, except for hydrogen, which only needs two electrons.
Common Mistakes to Avoid
When drawing Lewis structures, there are a few common mistakes to avoid: * Forgetting to check the octet rule * Not counting the total number of valence electrons correctly * Drawing multiple bonds between atoms that are not possible * Not showing lone pairs of electrons
Table of Common Lewis Structures

Here is a table of some common Lewis structures:
| Molecule | Lewis Structure |
|---|---|
| Water (H2O) |  |
| Methane (CH4) |  |
| Ammonia (NH3) |  |

In summary, Lewis structures are a powerful tool for understanding the molecular structure and properties of a compound. By following the steps outlined in this article and practicing with the worksheet, you should now be able to draw Lewis structures with confidence. Remember to always check the octet rule and avoid common mistakes. With practice and patience, you will become proficient in drawing Lewis structures and be able to apply this knowledge to a wide range of chemical concepts.
What is the purpose of Lewis structures?

+
The purpose of Lewis structures is to represent the bonding between atoms in a molecule and to predict the properties and behavior of the molecule.
How do I determine the total number of valence electrons in a molecule?

+
To determine the total number of valence electrons in a molecule, you need to count the number of valence electrons for each atom in the molecule and add them together.
What is the octet rule?

+
The octet rule states that each atom should have a full outer energy level, except for hydrogen, which only needs two electrons.