Lewis Structure Worksheet Answer Key: Chemistry Help

Mastering Lewis Structures: A Comprehensive Guide

When it comes to understanding chemical bonding, Lewis structures serve as a foundational tool in chemistry education. They provide a simple visual representation of how atoms bond to form molecules. This guide will help you master the art of drawing Lewis structures and understanding their significance in predicting molecular shapes, polarity, and reactivity.
Understanding Lewis Structures
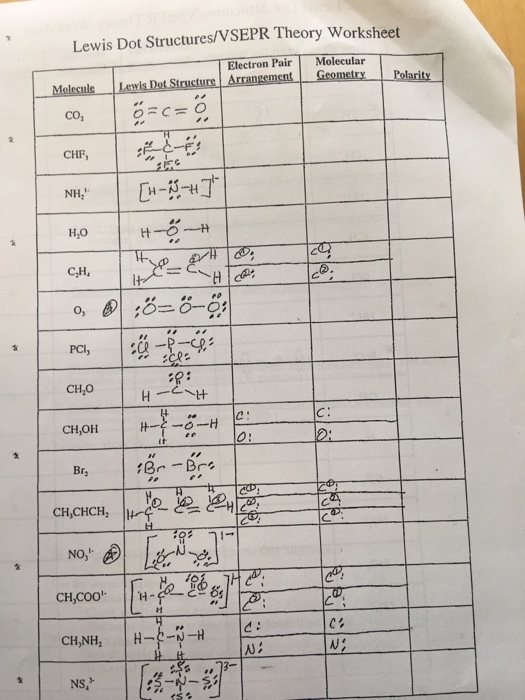
Before diving into the how-to of drawing Lewis structures, it's essential to understand what they represent:
- Atoms are shown with their symbol surrounded by dots for valence electrons.
- Shared pairs of electrons are represented by lines or dots between atoms.
- Unshared electrons (lone pairs) are shown as dots on the atom itself.
A Lewis structure provides a quick snapshot of the electron distribution in a molecule or ion:
| Symbol | Meaning |
HO O |
Shared electron pair (bond) |
HO with lone pair |
Lone electron pair |

Steps to Draw a Lewis Structure

Here is a detailed step-by-step guide to drawing Lewis structures:
- Determine the total number of valence electrons: Add up the valence electrons for each atom, adjusting for charge if dealing with ions.
- Identify the central atom: Generally, the least electronegative atom or the one with the least valence electrons in the group should be the central atom.
- Connect atoms with single bonds: Start by placing one electron pair between the central atom and each surrounding atom.
- Complete octets: Add electrons in pairs around the peripheral atoms until they have eight (an octet).
- Central atom octet: Then distribute remaining electrons to the central atom, striving for an octet unless the element is an exception (e.g., boron often has six).
- Check for formal charge: Ensure formal charges are minimized or as reasonable as possible.
- Check for resonance structures: If different arrangements of electrons lead to valid structures, draw all possible resonance structures.
📝 Note: Always verify that the total number of valence electrons used equals the initial count.
Practice Problems with Answer Key

Let's practice with some common molecules:
Example 1: Carbon Dioxide (CO2)

- Count total valence electrons: C (4) + 2 x O (12) = 22
- Identify central atom: Carbon
- Place carbon in the center, connected to two oxygens with single bonds.
- Add electrons to complete octets:
- Carbon needs 4 electrons to complete its octet (already has 4 from bonds), so add 4 more electrons.
- Oxygen atoms each need 6 electrons to complete octets (they each have 2 from bonds), so add 6 electrons to each oxygen.
- You'll notice that you've used up all the valence electrons without satisfying the octet rule on carbon. This indicates that carbon forms double bonds with oxygen:
[Image of CO2 Lewis Structure would go here]
Example 2: Ammonia (NH3)
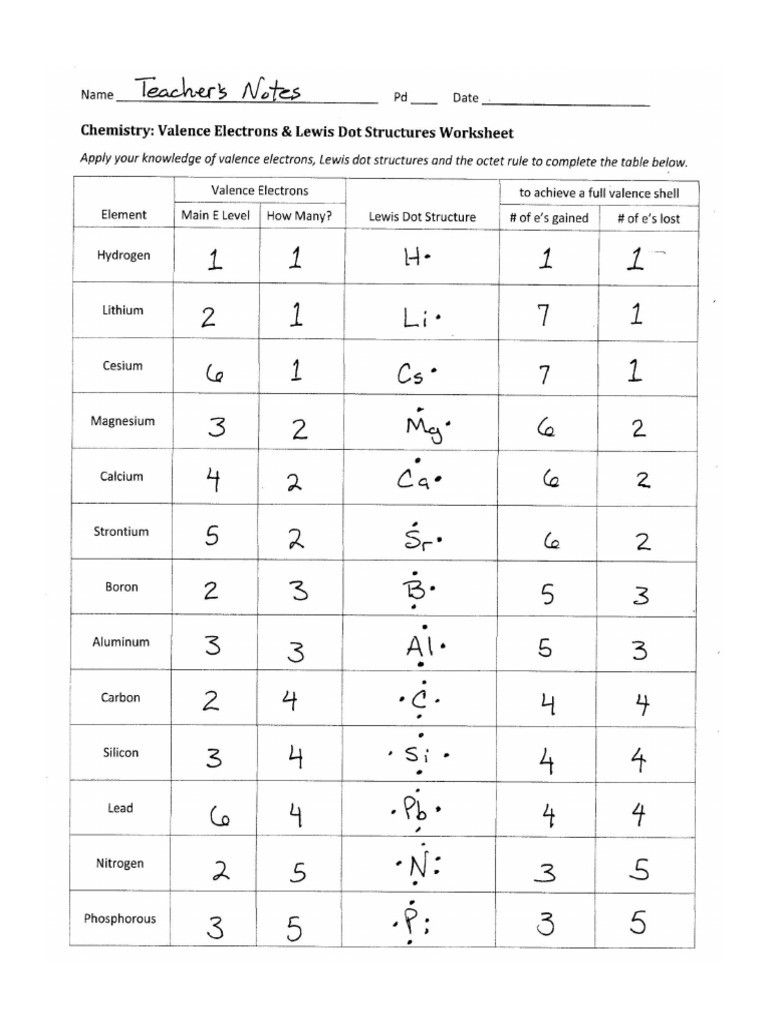
- Count total valence electrons: N (5) + 3 x H (3) = 8
- Identify central atom: Nitrogen
- Connect nitrogen to each hydrogen with single bonds.
- Add electrons to complete octets:
- Hydrogen atoms are already full with 2 electrons each.
- Nitrogen needs 6 more electrons for an octet, so add 6 electrons as lone pairs.
[Image of NH3 Lewis Structure would go here]
Example 3: Sulfate Ion (SO42-)

- Count total valence electrons: S (6) + 4 x O (32) + 2 for charge = 40
- Identify central atom: Sulfur
- Connect sulfur to each oxygen with single bonds.
- Add electrons to complete octets:
- Sulfur needs 6 electrons for an octet, which it now has from bonds.
- Each oxygen atom needs 4 electrons for an octet (since it has 2 from the bond already), so add 12 electrons in pairs to each oxygen.
- This structure uses up 32 electrons; the remaining 8 are added as lone pairs on sulfur to get the desired charge.
[Image of SO4^2- Lewis Structure would go here]
Wrapping Up
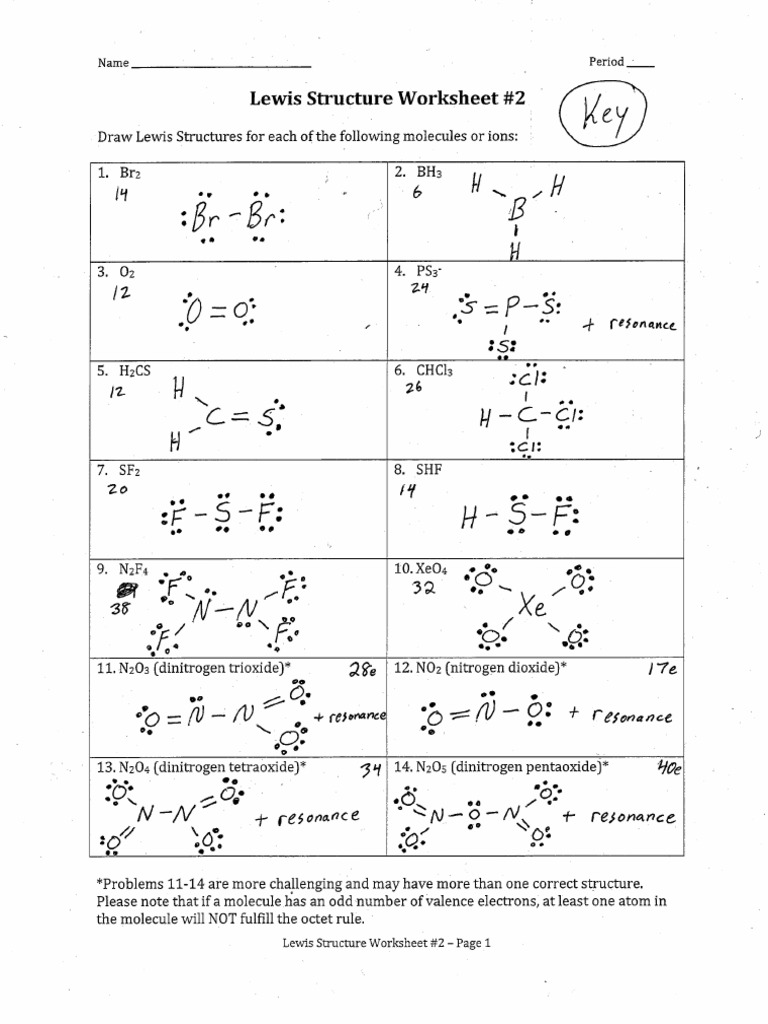
By following these steps and practicing with different molecules, you can master drawing Lewis structures effectively. They are not just a method for counting electrons but are key in understanding molecular behavior, polarity, and much of organic chemistry. Your ability to visualize electron distribution in molecules will be instrumental in predicting reactivity and physical properties.
Why are Lewis structures important in chemistry?

+
Lewis structures offer a simplified view of how atoms share or transfer electrons in a molecule, aiding in the prediction of molecular geometry, bonding, polarity, and chemical reactivity.
What if an atom cannot have an octet?
+
Some atoms like hydrogen (which needs only two electrons for a full shell) and elements like boron or beryllium (which can have less than eight electrons) are exceptions to the octet rule. Additionally, elements in periods 3 and beyond can have an expanded octet.
How do I deal with formal charges?

+
Formal charges should be minimized. They help identify the most stable arrangement of electrons in the structure, with a preference for structures where atoms have a formal charge of zero or as close to zero as possible.