Lewis Dot Structure Answers: 5 Essential Worksheets
Mastering the Lewis dot structure can significantly enhance your understanding of molecular chemistry. This form of diagrammatic representation aids in visualizing the electron arrangement in molecules, making it an indispensable tool for both students and professionals in the field of chemistry. Here are 5 essential worksheets tailored to provide practical experience and deepen your knowledge in crafting accurate Lewis structures.
Understanding the Basics of Lewis Dot Structures

Lewis dot structures, or electron dot diagrams, are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Here’s what you need to keep in mind:
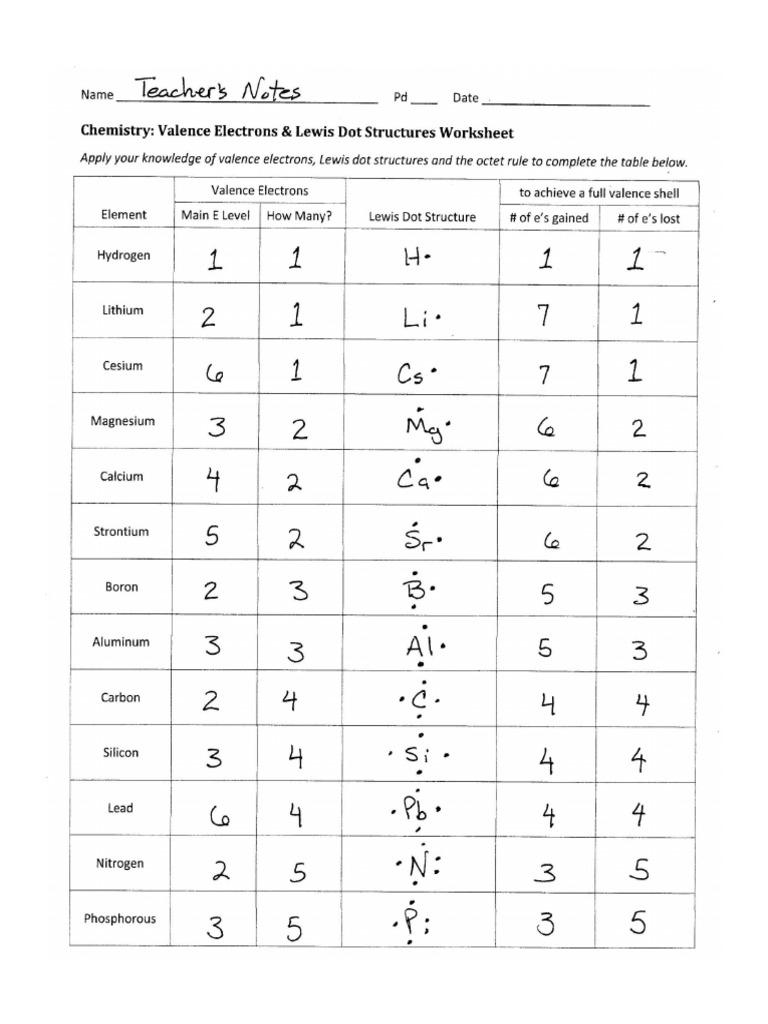
- Valence Electrons: Determine the number of valence electrons each atom has. This can be found from the periodic table.
- Octet Rule: Most atoms aim to have 8 electrons in their outermost shell, except for hydrogen which aims for 2.
- Bonds and Lone Pairs: Electron pairs are either bonding (forming bonds) or non-bonding (lone pairs).
Worksheet 1: Single Atom Practice

Start with individual atoms. Here are a few exercises to help you:
| Atom | Lewis Structure |
|---|---|
| Hydrogen (H) | |
| Helium (He) |
Work through the periodic table, focusing on the first row and then expanding to include elements from different groups.
Worksheet 2: Diatomic Molecules

Now, let’s move on to diatomic molecules. Here’s a table to guide you:
| Molecule | Lewis Structure |
|---|---|
| H2 | |
| N2 |
Worksheet 3: Simple Compounds

In this worksheet, practice with more complex molecules:
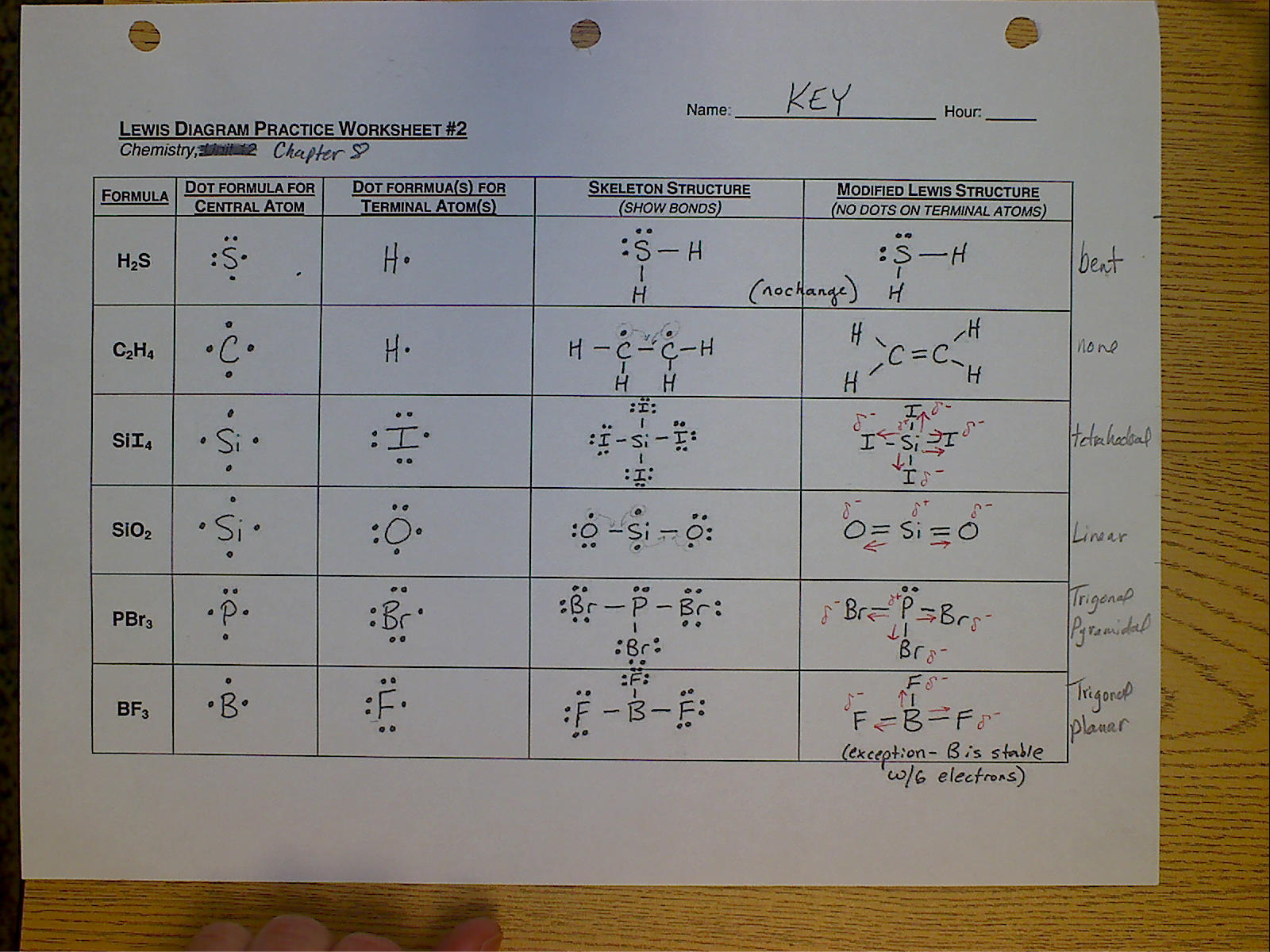
- Water (H2O): The oxygen atom should have two lone pairs.
- Carbon Dioxide (CO2): Both oxygen atoms are double-bonded to carbon, with no lone pairs on carbon.
- Ammonia (NH3): Nitrogen should have one lone pair and three bonds with hydrogen.
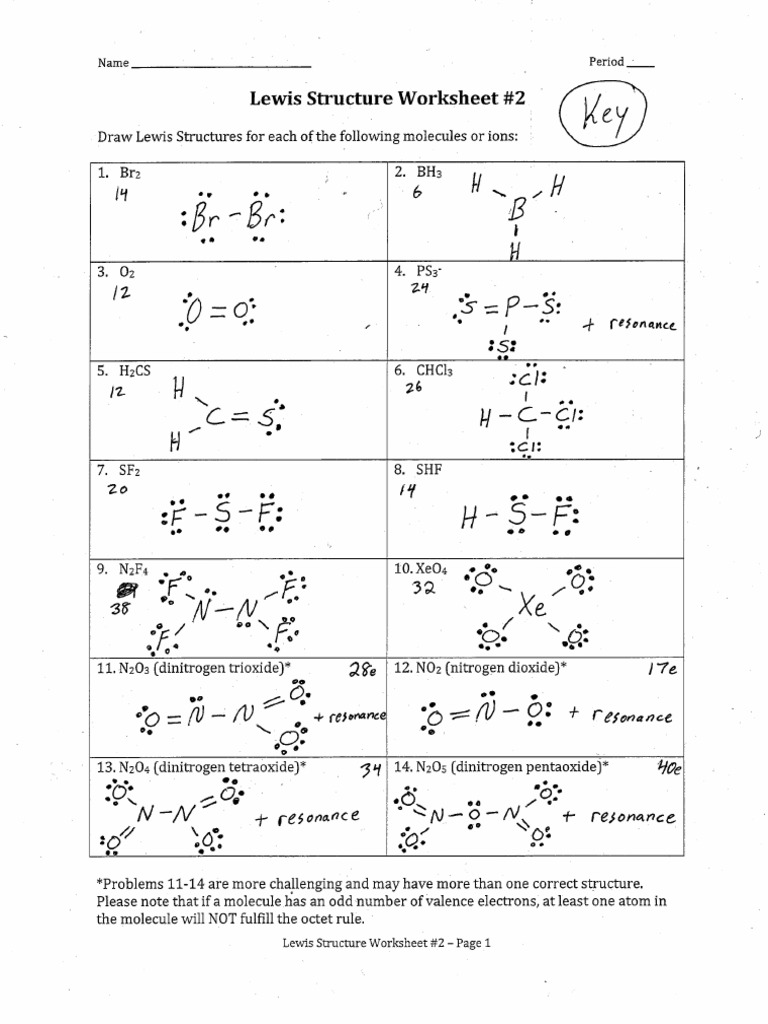
Worksheet 4: Resonance Structures

Resonance structures occur when there is more than one valid Lewis structure for a molecule. Here’s an example:
- Ozone (O3): One structure shows a single and a double bond between oxygen atoms, while another shows a different arrangement.
Practice finding resonance structures for:
- Carbonate ion (CO3^2-)
- Nitrate ion (NO3^-)
Worksheet 5: Expanded Octet and Odd Electron Species

Some molecules violate the octet rule:
- Phosphorus Pentachloride (PCl5): P has ten electrons in its valence shell.
- Odd Electron Molecules like Nitric Oxide (NO): Here, nitrogen has seven electrons, and oxygen has eight.
These worksheets should help you:
- Understand how to count valence electrons and distribute them among atoms.
- Recognize and depict the octet rule and its exceptions.
- Practice drawing Lewis structures for a variety of molecules, from simple to complex.
- Identify resonance structures where applicable.
💡 Note: Remember to check for formal charge distribution to ensure that the most stable structure is depicted when there is more than one possible arrangement of electrons.
In summary, mastering Lewis dot structures is crucial for understanding chemical bonding. By practicing these worksheets, you will gain confidence in constructing these diagrams, appreciating molecular geometry, and predicting the reactivity of molecules. Understanding these concepts not only enhances your grasp on the basics of chemistry but also prepares you for more advanced topics in the field.
What is the octet rule?
+
The octet rule is a chemical principle that states atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell, similar to the noble gases.
How do you determine the number of valence electrons?

+
Valence electrons can be found by looking at the group number in the periodic table. For main group elements, this number corresponds to the number of valence electrons, except for helium which has two.
Why are resonance structures important?
+
Resonance structures provide a way to represent the delocalization of electrons within a molecule. They give us insight into molecular stability, reactivity, and the overall electron distribution, making it crucial for understanding many chemical properties.