5 Tips for Mastering Lewis Dot Structure Worksheets
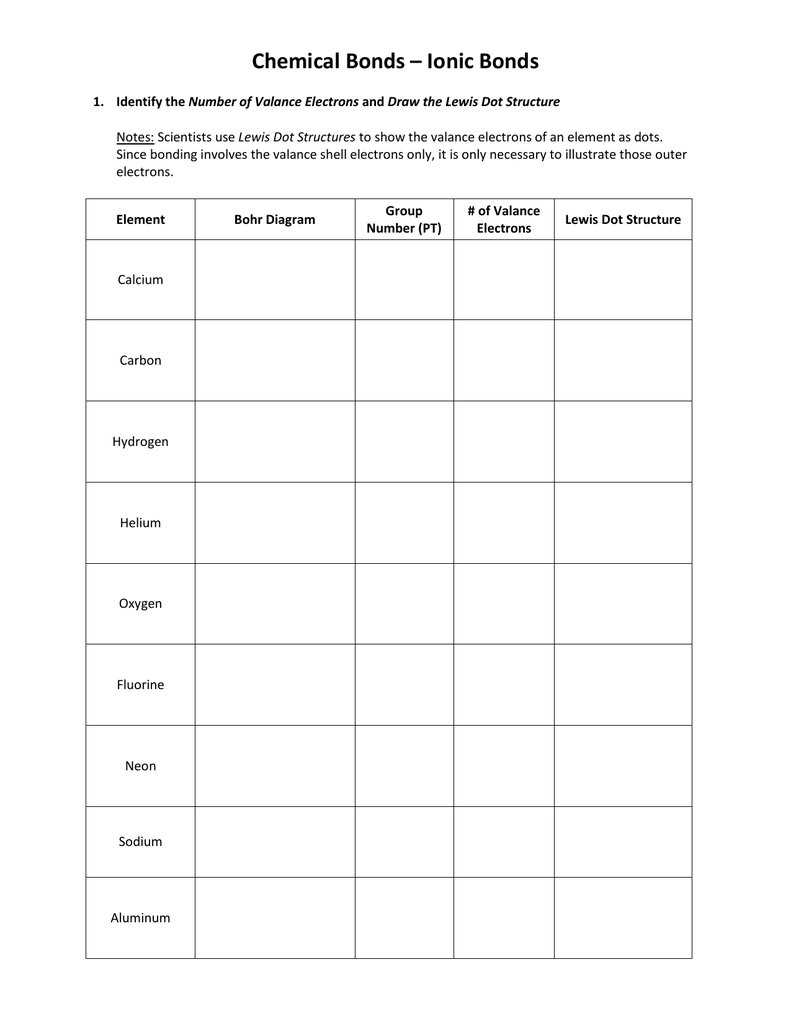
In the realm of chemistry, understanding molecular structure and bonding is essential for students and professionals alike. One of the tools used to visualize this is the Lewis dot structure. These diagrams offer a clear and straightforward way to represent the valence electrons of atoms, helping to predict the molecular shape, polarity, and chemical properties of substances. Here, we explore five practical tips that will guide you in mastering the construction and interpretation of Lewis dot structure worksheets, ensuring that you not only solve the problems correctly but also deeply understand the underlying chemical concepts.
The Basics of Lewis Dot Structures

Before diving into the tips, let’s briefly review what a Lewis dot structure entails:
- It shows the atoms and the valence electrons in a molecule.
- Each dot represents one valence electron, and they are placed around the element symbol.
- Bonds are represented by lines (single, double, or triple bonds).
- Lone pairs are depicted as pairs of dots.
An image here would illustrate a simple molecule like H2O to visually aid understanding of these points.
Tip 1: Start with Counting Valence Electrons

The first step in constructing a Lewis dot structure is to determine the number of valence electrons for each atom:
- Identify the element and its position in the periodic table.
- Count the number of valence electrons based on the group number.
Example: For oxygen in H2O, oxygen has 6 valence electrons. The two hydrogen atoms contribute 2 more electrons, making a total of 8 electrons to distribute in the structure.
🧐 Note: For molecules with a net charge, adjust the total electron count accordingly.
Tip 2: Understand the Octet Rule and Exceptions

The octet rule states that atoms tend to achieve a noble gas configuration by sharing, losing, or gaining electrons to have 8 valence electrons, except for:
- Hydrogen and Helium, which aim for 2 electrons.
- Elements like Beryllium and Boron, which can have incomplete octets.
- Elements from the third row and beyond, which can have expanded octets.
This rule is not universal, and exceptions provide opportunities for advanced chemical understanding.
Tip 3: Master the Art of Placing Electrons

Placing electrons in the right positions involves:
- Placing atoms bonded to the central atom first.
- Distributing the remaining electrons to fulfill the octet rule.
- Creating bonds if atoms do not have a full octet.
| Molecule | Electrons Distribution | Resulting Structure |
|---|---|---|
| H2O | 1 O in center, 2 H attached | H-O-H (with 4 lone pairs on O) |
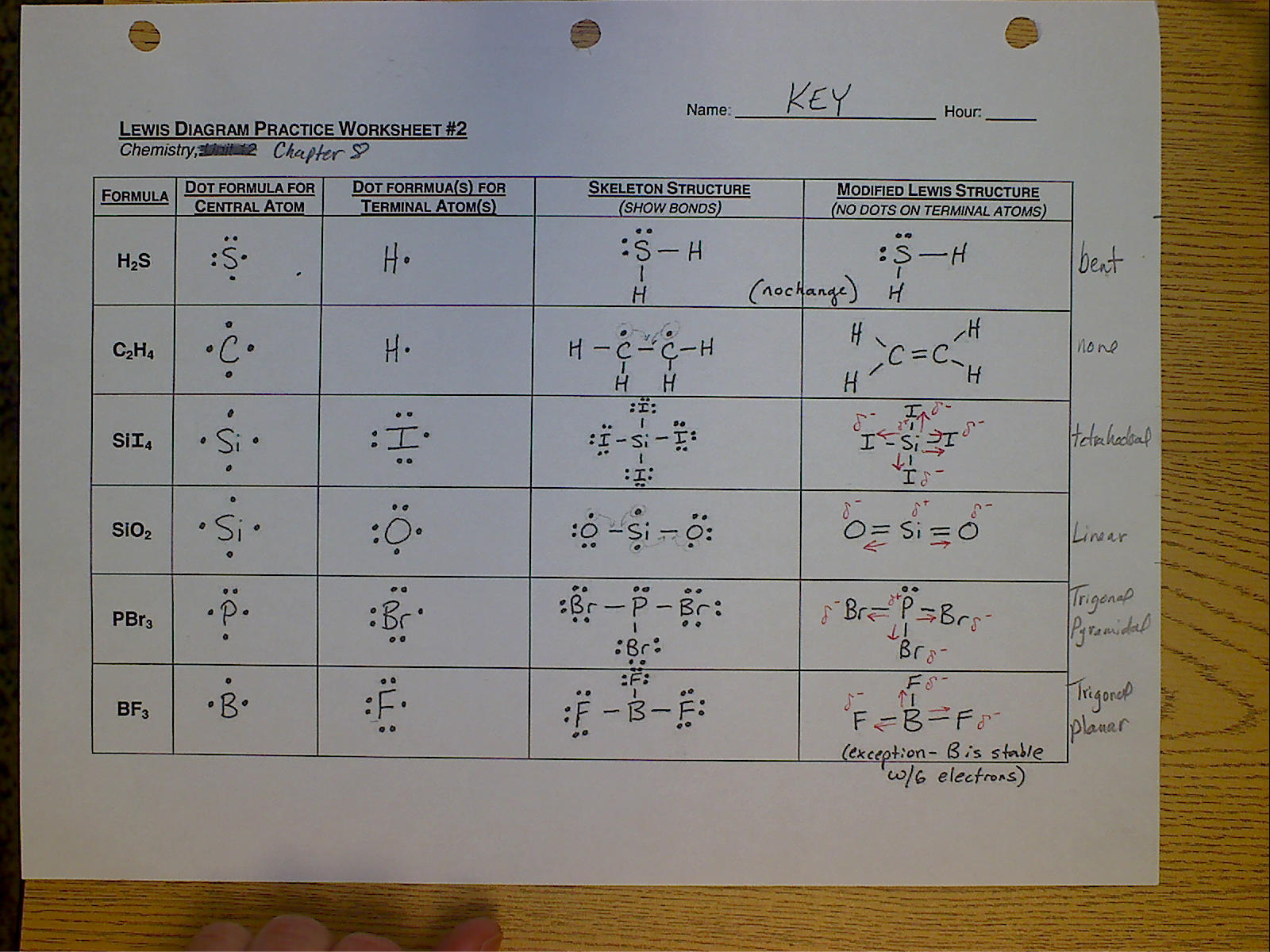
Tip 4: Check for Formal Charges

Formal charges help in determining the most stable structure:
- Formula: FC = V - (B + L/2)
- Where V is the number of valence electrons, B is the number of bonds, and L is the number of lone pairs.
Minimizing formal charges helps predict more likely structures:
💡 Note: The most stable Lewis structure often has the smallest formal charges, with no atom bearing a charge greater than 1 if possible.
Tip 5: Use Molecular Geometry for Visual Clarity

Understanding the molecular geometry can help in predicting bond angles, positions of lone pairs, and the overall shape of the molecule:
- VSEPR theory (Valence Shell Electron Pair Repulsion) can aid in visualizing the structure.
- Draw out the molecule in three dimensions if possible for better understanding.
In wrapping up, mastering Lewis dot structures involves more than just counting electrons and drawing lines. It requires a deep understanding of chemical principles like the octet rule, formal charges, and molecular geometry. By following these five tips, you'll not only become adept at constructing these diagrams but also gain insights into the behavior of molecules. This knowledge forms the backbone of chemistry education, helping students predict reactivity, understand molecular properties, and navigate the complex world of chemical bonding and reactions.
What is the purpose of drawing Lewis dot structures?

+
Lewis dot structures help in visualizing the sharing or transfer of valence electrons between atoms, providing insights into the molecular shape, bonding, and potential reactivity of compounds.
How do I handle elements that don’t follow the octet rule?

+
For elements like boron, which often forms stable compounds with an incomplete octet, or those in period 3 or beyond that can have an expanded octet, you should recognize these exceptions and place electrons accordingly, ensuring the formal charges make sense.
Can a molecule have more than one acceptable Lewis structure?

+
Yes, some molecules can have resonance structures, where electrons are delocalized over the molecule, resulting in multiple correct Lewis structures. These structures contribute to the overall stability and properties of the molecule.
Why are formal charges important in Lewis structures?

+
Formal charges help determine the most probable arrangement of electrons, predicting which structure is most likely to occur in nature, thereby contributing to the understanding of a molecule’s reactivity and stability.