Master Lewis Dot Diagrams with Our Chem Worksheet 5 7
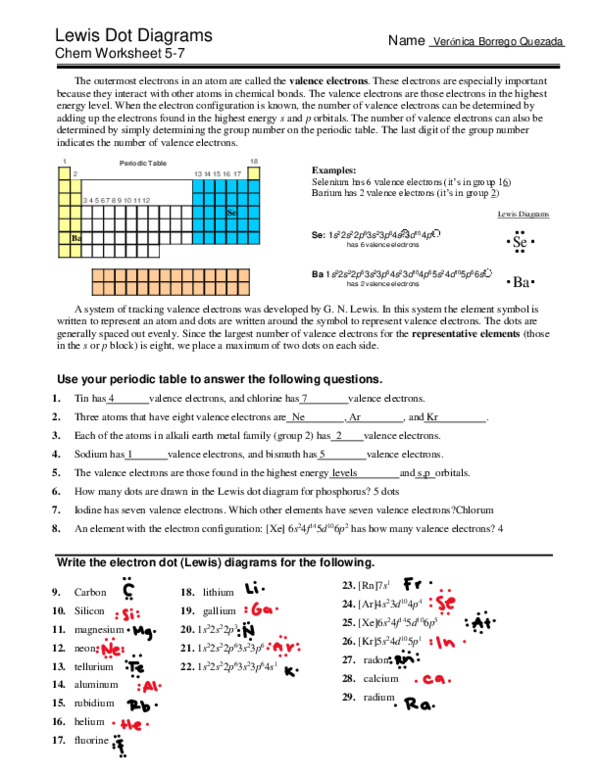
In the fascinating world of chemistry, understanding molecular structures is not just a fundamental aspect of the subject, but also a gateway to grasping more complex chemical theories and phenomena. One pivotal tool in achieving this understanding is the Lewis Dot Diagram. Used to represent the valence electrons in an atom, these diagrams help chemists visualize the bonding in molecules, predict reactivity, and understand the shape of molecules. This blog post delves into mastering Lewis Dot Diagrams through Chem Worksheet 5 7, a guide that offers a step-by-step approach to constructing these diagrams effectively.
Understanding Lewis Dot Diagrams


Lewis Dot Diagrams, also known as electron dot structures or Lewis structures, are simple yet powerful representations designed to indicate:
- The number of valence electrons an atom has.
- How atoms share or transfer these electrons to achieve a stable electron configuration.
- The formal charges on atoms within a molecule.
Here’s how you can construct a Lewis Dot Diagram:
- Determine the total number of valence electrons: Find the group number of each element in the periodic table. For example, carbon is in Group 4, meaning it has four valence electrons.
- Write the skeletal structure: Start by drawing the atoms in the molecule. The central atom usually has the lowest electronegativity or is the least frequent element.
- Place electrons: First, place a pair of electrons between each bonded atom to form single bonds. Then, distribute the remaining electrons to achieve an octet for each atom except hydrogen which only needs two electrons.
- Check for octet rule satisfaction: If an atom lacks an octet, consider multiple bonding or resonating structures.
Utilizing Chem Worksheet 5 7

Chem Worksheet 5 7 is designed as a practice tool to enhance your proficiency in drawing Lewis Dot Diagrams:
Step-by-Step Guide

The worksheet breaks down the process into manageable steps:
- Element Identification: Identify the elements in the molecule.
- Valence Electrons Count: Determine how many valence electrons each atom brings to the table.
- Forming Bonds: Begin with single bonds, adding electrons to form these bonds and continue adding electrons until you’ve used all the valence electrons or atoms have octets.
- Octet Rule Compliance: Ensure that each atom (except H, He, Li, Be) has an octet of electrons, adjusting the bond order if necessary.
- Formal Charges: Calculate formal charges to assess which structure is more plausible.
Here’s a simple table for quick reference:
| Element | Valence Electrons | Octet Rule Requirement |
|---|---|---|
| H | 1 | 2 |
| He | 2 | 2 |
| Li, Be | 1, 2 | 2 |
| C, N, O, F | 4, 5, 6, 7 | 8 |

Practicing with Chem Worksheet 5 7
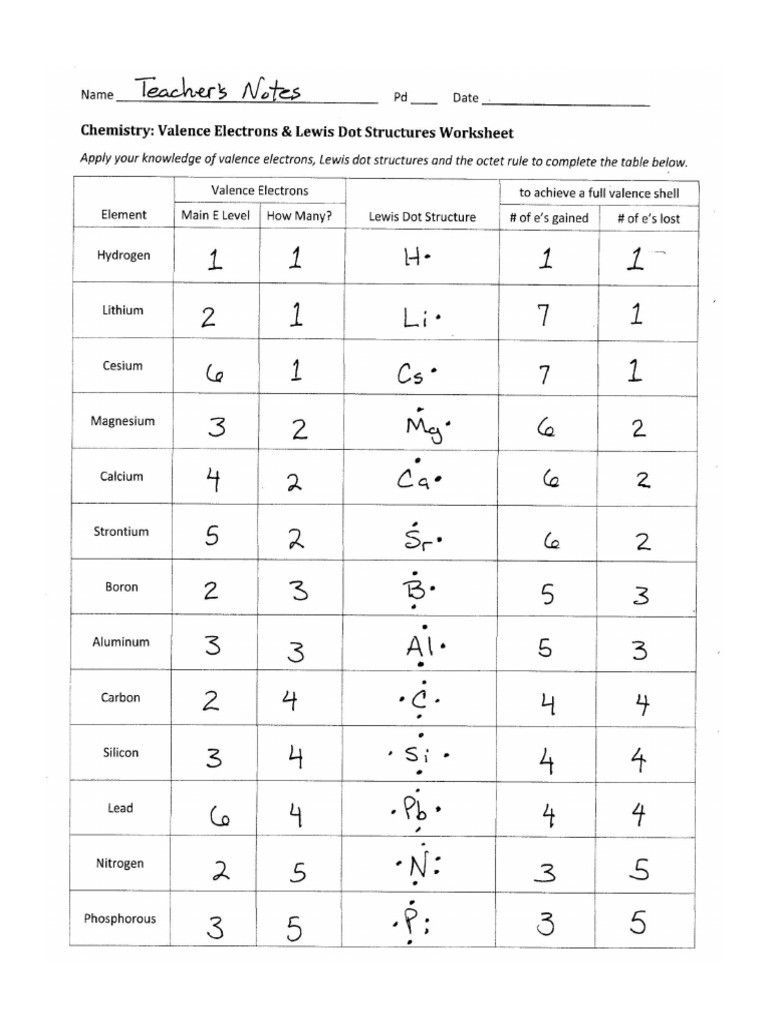
Engage with a series of exercises where you draw Lewis Dot Diagrams for various molecules:
- H2O
- NH3
- CO2
- CH4
- C2H6
Each molecule presents different challenges in applying the Lewis Dot Diagram rules, helping you gain a deeper understanding of electron sharing and molecular structures.
🔍 Note: Remember, the octet rule can be broken in some instances, especially for elements in periods beyond the second row or for highly electronegative atoms like boron or beryllium.
Applications of Lewis Dot Diagrams

Lewis Dot Diagrams are not just academic exercises. They have practical applications in various fields of chemistry:
- Organic Chemistry: Predicting reaction mechanisms, understanding molecular interactions, and drug design.
- Inorganic Chemistry: Understanding the structure of coordination compounds and crystal field theory.
- Biochemistry: Analyzing the structure and function of biomolecules like proteins and nucleic acids.
- Environmental Chemistry: Predicting the reactivity of pollutants and their interactions with biological systems.
🛑 Note: While Lewis Dot Diagrams provide a simplified view, remember that real molecules are more complex and might involve three-dimensional considerations not captured by a two-dimensional diagram.
The final recap of our journey into mastering Lewis Dot Diagrams through Chem Worksheet 5 7 highlights how these diagrams serve as essential tools for visualizing electron distribution, understanding bonding, and predicting molecular properties. The worksheet offers a practical way to internalize these concepts, providing exercises that range from simple to complex, helping students grasp the nuances of electron-sharing in molecules. Remember, practice not only helps in mastering the technique but also in improving your confidence in tackling more intricate chemical structures and bonding scenarios.
Why are Lewis Dot Diagrams important in chemistry?

+
Lewis Dot Diagrams are crucial because they provide insight into the bonding, molecular shape, and chemical reactivity. They help predict how molecules will interact with each other, how they might react, and even their physical properties like solubility and polarity.
How do you know when to use double or triple bonds in Lewis Diagrams?

+
Double or triple bonds are used when an atom cannot achieve an octet with just single bonds. This often happens with atoms like carbon, nitrogen, and oxygen which have more than four valence electrons. You add more bonds to satisfy the octet rule for as many atoms as possible.
Can you show a formal charge on a Lewis Dot Diagram?
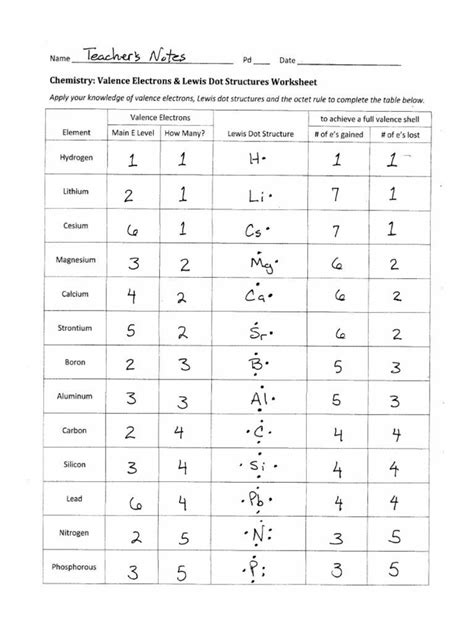
+
Yes, you can depict formal charges in Lewis Dot Diagrams. They are important for identifying the most plausible resonance structures or for predicting the stability and reactivity of molecules. Formal charge is calculated as the number of valence electrons minus the number of nonbonding electrons minus half the number of bonding electrons.