Lewis Dot Diagram Answers: Master the Worksheet Easily

How to Draw Lewis Dot Diagrams: A Step-by-Step Guide

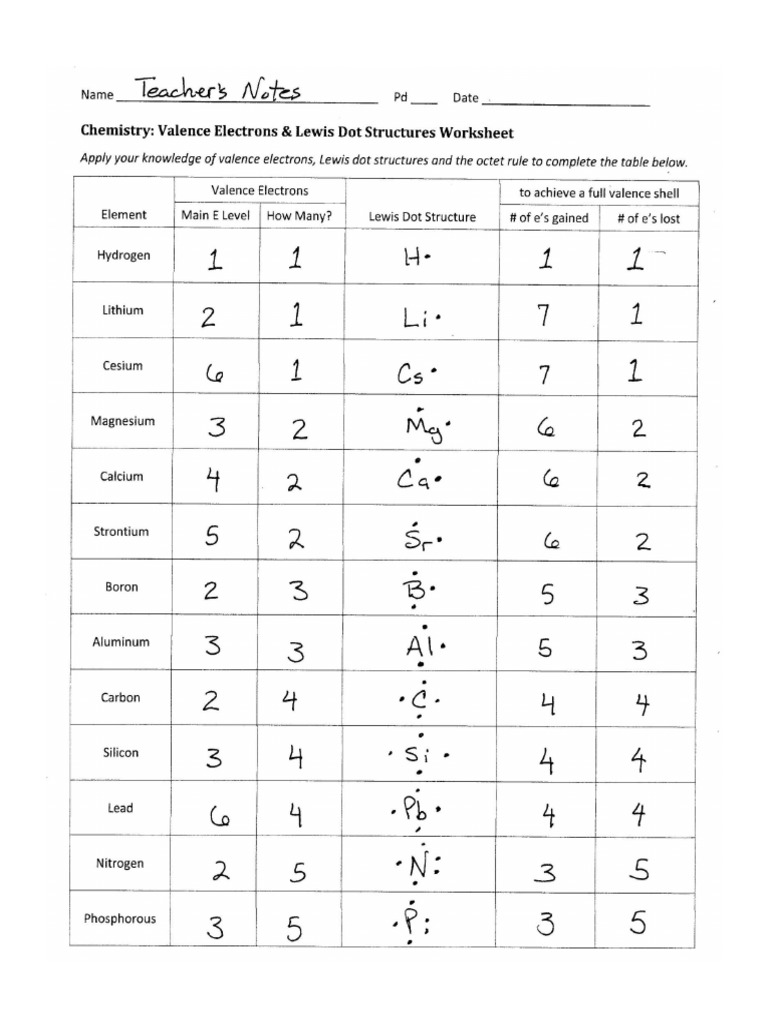
Understanding Lewis dot diagrams is essential for mastering chemistry, especially when it comes to visualizing the valence electrons of atoms and understanding their bonding. This guide will walk you through the process of drawing Lewis dot diagrams with clear, concise steps and offer some helpful tips to tackle worksheets with ease.
What Are Lewis Dot Diagrams?

Lewis dot diagrams, named after the American chemist Gilbert N. Lewis, represent the valence electrons of atoms using dots placed around the element symbol. Here's a breakdown:
- Each dot represents one valence electron.
- The number of dots must correspond to the element's group number in the periodic table.
- Valence electrons are arranged in pairs or alone, depending on their availability.
Steps to Draw a Basic Lewis Dot Diagram

Follow these steps to create a Lewis dot diagram for an atom:
1. Find the Atomic Number and Identify the Element
Start with the atomic number of the element. This tells you the number of electrons and protons in an uncharged atom.
2. Determine the Valence Electrons
Find out the group number in the periodic table. The group number (excluding transition metals) directly relates to the number of valence electrons for the first two and last three periods:
| Group Number | Valence Electrons |
|---|---|
| 1 | 1 |
| 2 | 2 |
| 13 | 3 |
| 14 | 4 |
| 15 | 5 |
| 16 | 6 |
| 17 | 7 |
| 18 | 8 (Noble Gases have full valence shells, except Helium with 2) |

3. Place the Dots Around the Element Symbol
Here's how to place the dots:
- Start with one dot on each side of the symbol, going clockwise or counterclockwise.
- After each side has one dot, start pairing up dots until you reach the total number of valence electrons.
- Pairing is crucial as it signifies electrons that can form bonds or lone pairs in molecules.
📝 Note: For elements with many electrons, ensure that lone pairs and bonding pairs are distinguished in your diagram.
Advanced Techniques: Cations and Anions
Adjusting for ions:
- Cations (Positive Ions): When an atom loses electrons, it becomes a cation. Remove the corresponding number of dots from the diagram to reflect the lost electrons. E.g., Sodium (Na) loses one electron to become Na+, hence, the diagram shows only 0 dots.
- Anions (Negative Ions): When an atom gains electrons, it becomes an anion. Add the appropriate number of dots. For example, Chlorine (Cl) gains one electron to form Cl-, hence, the diagram shows 8 dots arranged as three pairs and one lone pair.
Common Mistakes to Avoid

Avoid these common pitfalls when drawing Lewis dot diagrams:
- Not pairing electrons: Each side of the symbol should have a maximum of two dots.
- Incorrect number of valence electrons: Always ensure the diagram matches the element's group number or consider the ion's charge.
- Forgetting to show charge for ions: Make sure to indicate the charge for cations and anions.
🔎 Note: Be mindful of how electrons are distributed to represent bonds and lone pairs correctly in complex molecules or ions.
In summary, mastering Lewis dot diagrams requires understanding the basic principles of atomic structure and electron arrangement. By following these steps, you can approach worksheet problems with confidence:
- Know the element’s group and its valence electrons.
- Distribute the dots around the symbol correctly.
- Adjust for ions by adding or subtracting electrons as necessary.
Now, you’re equipped with the knowledge to easily navigate Lewis dot diagram worksheets, providing a solid foundation for understanding chemical bonding and structure.
What’s the Purpose of Lewis Dot Diagrams?

+
Lewis dot diagrams help visualize the valence electrons of atoms, providing insights into how atoms bond to form molecules or compounds.
How Do You Know How Many Valence Electrons an Element Has?

+
The number of valence electrons correlates with the group number of the element in the periodic table (for groups 1-2 and 13-18).
Why Do We Use Pairs of Dots?

+
Pairing dots represents electron pairs, which are significant in forming bonds and completing octets or satisfying octet rule exceptions.