5 Steps to Master Lewis Dot Diagrams Easily
Understanding Lewis dot diagrams, also known as Lewis structures or electron dot structures, is fundamental for anyone delving into chemistry. These diagrams provide a simple way to represent the valence electrons of atoms and how they form bonds, which are the building blocks of molecular structures. Whether you're a student grappling with chemistry homework or a curious mind eager to understand the composition of matter, mastering Lewis dot diagrams is a crucial skill. Here are five straightforward steps to help you grasp this concept with ease and confidence.
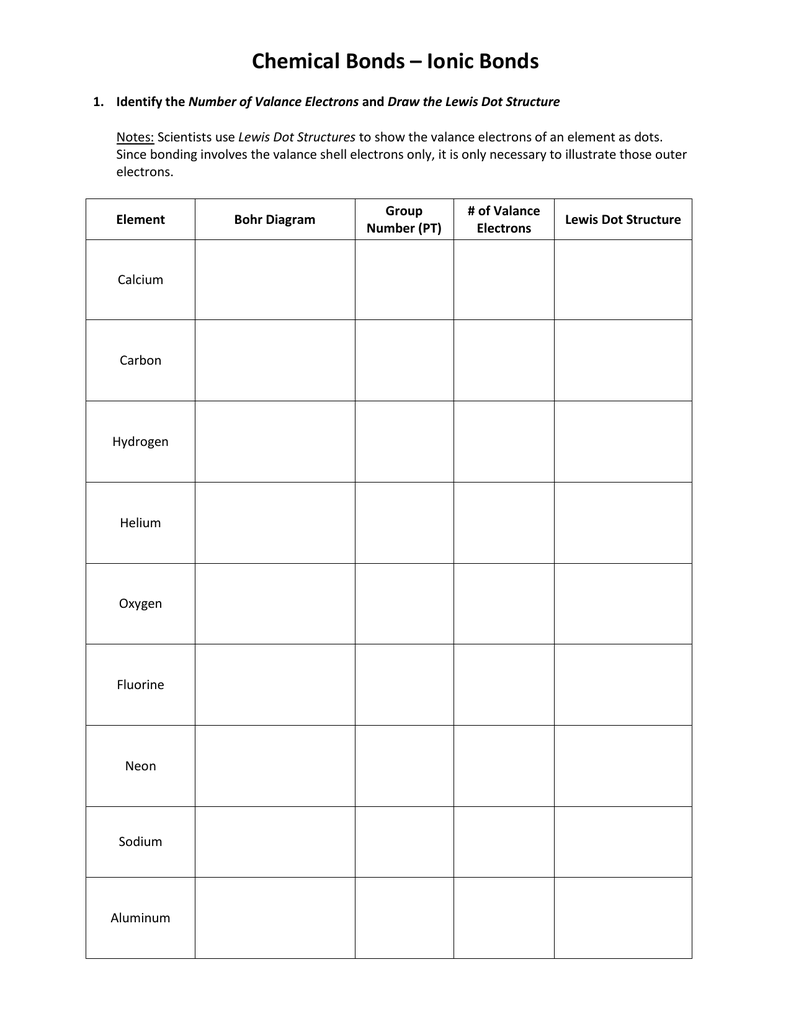
Step 1: Count Valence Electrons

The first step in drawing a Lewis dot diagram involves identifying the total number of valence electrons for the atom or molecule you are working with. Valence electrons are the electrons in the outermost energy level or shell of an atom, which are the electrons most involved in chemical reactions.
- Find the group number of the element from the periodic table. For example, Group 1 elements have 1 valence electron, Group 2 elements have 2, and so on.
- For non-metals, especially from Groups 15-17, add one electron for each negative charge in an ion.
- Subtract electrons for positive charges in ions.
Here’s a simple example:
| Element | Group Number | Valence Electrons |
|---|---|---|
| Carbon © | 14 | 4 |
| Oxygen (O) | 16 | 6 |

💡 Note: Remember that the number of valence electrons influences how an atom will bond.
Step 2: Determine Central Atom

Once you’ve counted the valence electrons, the next step is to identify which atom should be the central atom. This is typically:
- The least electronegative atom if all elements are from the same group.
- An element from Group 14, like Carbon or Silicon, for organic compounds.
- The element with the lowest electronegativity excluding Hydrogen (H) in most cases.
Step 3: Arrange Atoms and Draw Bonds

With the central atom decided, you’ll arrange the atoms around it, typically placing hydrogen atoms (which can only bond once) on the edges. Here’s how to proceed:
- Draw the central atom.
- Place the other atoms around it, creating single bonds.
- Count the total bonds made. Each bond represents two shared electrons.
Ensure that the number of bonds doesn’t exceed the valence capacity of the atoms involved.
Step 4: Place Remaining Electrons

After arranging the atoms and drawing the bonds, you need to place the remaining valence electrons around each atom, starting with the outer atoms:
- Outer atoms prefer to have an octet of electrons (8), except for hydrogen, which needs 2.
- Place pairs of electrons around the outer atoms first.
- If electrons are left, distribute them around the central atom.
📌 Note: The central atom might not always have an octet, especially if it’s an element from period 3 or below, which can have an expanded octet.
Step 5: Check and Adjust

The final step involves reviewing and adjusting your diagram to ensure:
- All atoms have an octet (except for hydrogen, which has a duet), unless it’s an exception like boron or beryllium, which can have incomplete octets.
- The total number of electrons in the diagram matches the number of valence electrons you calculated initially.
- Charges are appropriately assigned (if any) and the formal charges of atoms add up to the overall charge of the molecule or ion.
If you find yourself with an uneven electron count or if any atom does not have a full octet, you may need to add multiple bonds or adjust your initial placement of electrons.
💡 Note: When dealing with molecules or polyatomic ions, resonance structures might be necessary if the arrangement of electrons results in multiple valid structures.
In the journey of learning chemistry, Lewis dot diagrams are more than just a method for drawing; they represent the foundational understanding of how atoms bond and share electrons to form molecules. Mastery of these diagrams not only aids in visualizing chemical structures but also in predicting reactivity, understanding bonding patterns, and even deducing molecular shapes. Whether you're aiming to excel in a chemistry course or are just starting out, these steps will steer you towards a deeper appreciation of molecular science. With patience and practice, the once-intimidating electron dot structures will become a familiar friend in your chemical toolkit.
Why is counting valence electrons important in Lewis structures?

+
Valence electrons determine how an atom will bond with others to achieve a stable electron configuration. By understanding the number of valence electrons, you can predict the type of chemical bonds the atom will form.
Can Lewis structures always be drawn for all molecules?

+
Yes, Lewis structures can be drawn for most molecules, although some complex molecules, especially those with d-orbitals involved in bonding, might require more advanced models like molecular orbital theory.
How do I know which atom is the central atom in a molecule?

+
As a general rule, the central atom is often the least electronegative element that is not hydrogen. For organic compounds, carbon usually plays this role due to its unique bonding capabilities.