Master IUPAC Naming with Our Practice Worksheets

Mastering IUPAC (International Union of Pure and Applied Chemistry) nomenclature can be a daunting task for many students studying chemistry, but it is essential for understanding and communicating chemical structures effectively. The rules of IUPAC naming seem complex at first, but with practice, these can become second nature. This blog post will provide you with detailed guidance on how to approach IUPAC naming, complete with practice worksheets to sharpen your skills. Let's dive in!
Understanding IUPAC Nomenclature


The IUPAC system was developed to ensure chemical names are consistent, unique, and unambiguous. Here are the key components of IUPAC naming:
- Root Name: Indicates the longest continuous carbon chain in the molecule, forming the base name.
- Suffix: Indicates the functional group or the presence of multiple bonds (e.g., -ane for alkanes, -ene for alkenes).
- Prefixes: Denote substituents or branches off the main chain.
- Locants: Numbers indicating the positions of substituents or functional groups.
- Stereochemistry: Denotes the spatial arrangement of atoms around double bonds or chiral centers.
By mastering these elements, you can name virtually any organic compound according to IUPAC standards.
Step-by-Step Guide to Naming Organic Compounds

Here is a structured method to approach IUPAC naming:
Identify the Longest Carbon Chain

Begin by identifying the longest carbon chain in the molecule, which will give you the root name. Remember to consider:
- The chain must be continuous, and you should count the carbons in all possible directions.
- If there are multiple chains of equal length, select the one with the most substituents.
Name the Functional Groups or Multiple Bonds
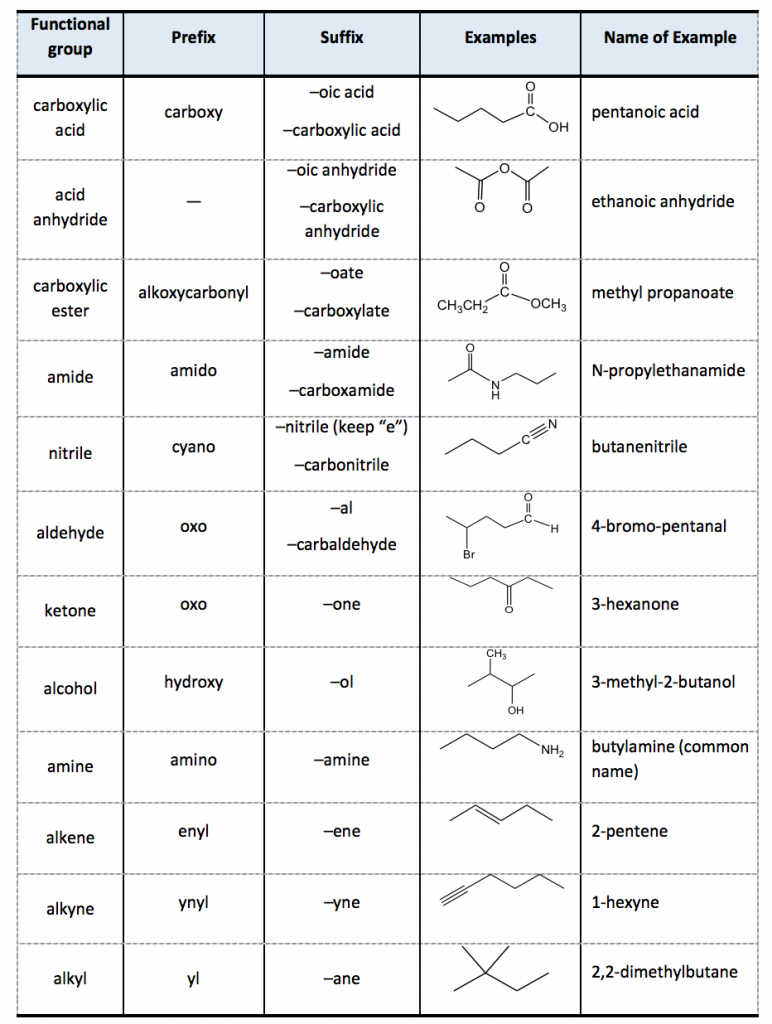
Next, identify any functional groups or double/triple bonds in the molecule:
- Choose the suffix according to the highest-priority functional group present.
- Use "-ol" for alcohols, "-al" for aldehydes, "-one" for ketones, etc.
- Indicate the position of the functional group with a number.
- If there are multiple functional groups, the one with the highest priority dictates the suffix.
Name Substituents
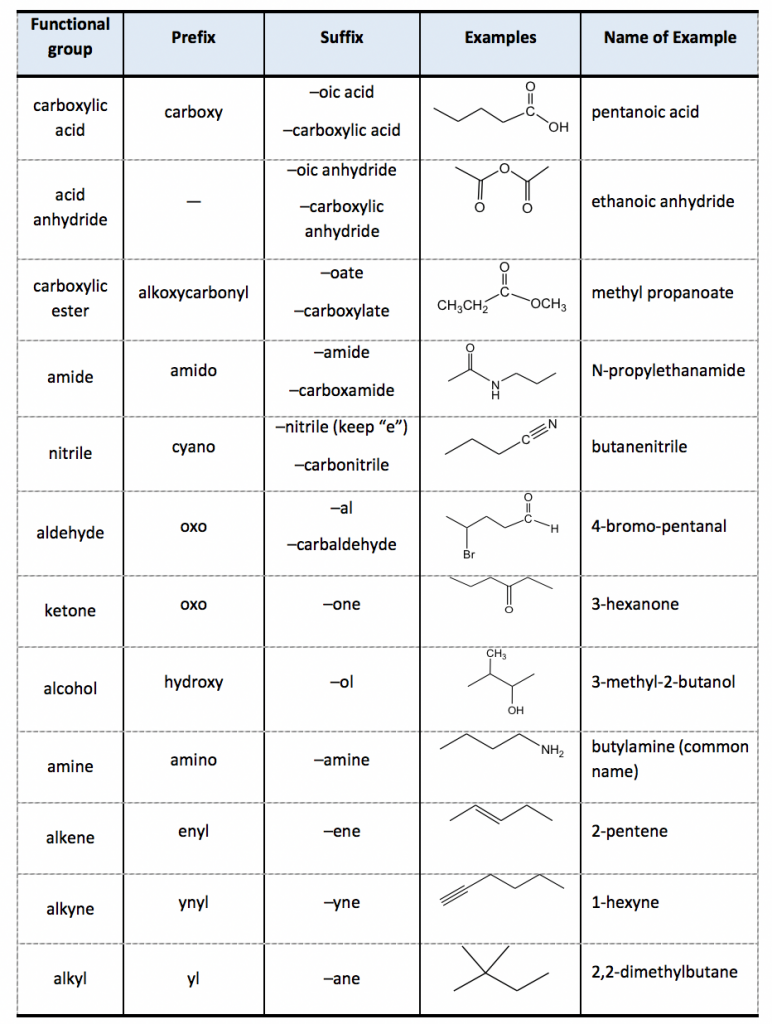
Substituents are groups attached to the main chain:
- Name the substituents by their length (e.g., "methyl", "ethyl").
- If there are multiple identical substituents, use prefixes like "di-", "tri-", "tetra-".
Number the Chain and Assign Locants

Number the carbon atoms in the chain to give the lowest possible numbers to:
- The substituents
- The functional group or double/triple bond
When listing substituents, arrange them alphabetically ignoring the prefixes:
- Example: 2-ethyl-3-methylpentane
Include Stereochemistry When Applicable

Stereochemical designations like Z/E (cis/trans) or R/S must be included:
- For double bonds, use Z (Zusammen) or E (Entgegen) based on the Cahn-Ingold-Prelog priority rules.
- For chiral centers, determine the R or S configuration.
Practice Worksheets for IUPAC Naming

To reinforce your understanding, here are some practice worksheets:
| Exercise | Description |
|---|---|
| Basic Alkanes | Naming straight-chain alkanes from 1 to 10 carbons. |
| Substituted Alkanes | Introducing substituents to alkanes. |
| Alkenes and Alkynes | Naming compounds with double and triple bonds. |
| Cyclic Compounds | Naming cyclic structures with or without substituents. |
| Functional Groups | Naming compounds with alcohol, aldehyde, ketone, carboxylic acid, and ester groups. |
| Stereochemistry | Naming compounds with stereochemical considerations. |

💡 Note: Don't just memorize names. Practice constructing the names to understand the logic behind IUPAC rules.
Advanced IUPAC Naming Techniques

Once you're comfortable with the basics, here are some advanced techniques to tackle more complex molecules:
Polyfunctional Compounds

When dealing with multiple functional groups:
- Identify the principal functional group and name it first with its locant.
- Then name the remaining groups as substituents.
- Use the appropriate prefixes and suffixes.
Ring Structures

Naming cyclic compounds requires specific rules:
- If a ring has a greater number of substituents, it might be preferable to use it as the principal chain.
- Number the ring starting from the functional group or the substituent that appears first in alphabetical order.
Bridged Bicyclic Compounds

Naming bridged bicyclic compounds involves recognizing the bridge system:
- Determine the bridgehead positions.
- Count the number of carbons in each bridge.
- Arrange the numbers of carbons in the bridges in descending order, separated by dots, as a prefix to the name.
Overcoming Common Mistakes in IUPAC Naming

Students often make similar mistakes when learning IUPAC nomenclature. Here are some common pitfalls to avoid:
- Incorrect chain length: Always choose the longest chain, even if it branches.
- Misnumbering: Ensure the substituents and functional groups are numbered to give the lowest possible combination of locants.
- Neglecting stereochemistry: Missing Z/E or R/S designations can lead to incorrect naming.
- Confusing prefixes: Be careful with prefix order, especially when using "di-", "tri-", etc., and the alphabetic listing.
📝 Note: Always verify your work by cross-checking your named compounds with a reliable source to ensure accuracy.
By using practice worksheets and understanding the systematic approach to IUPAC naming, you'll find the process becoming more intuitive over time. Regular practice will not only help you name compounds accurately but will also enhance your ability to recognize chemical structures and predict their properties. Remember, the key to mastering IUPAC nomenclature is consistency in application and understanding the underlying logic. Whether you're a student preparing for exams or a professional working in a chemistry-related field, these skills will serve you well in communicating and understanding the science of molecules.
Why is IUPAC nomenclature important in chemistry?

+
IUPAC nomenclature provides a systematic way to name chemical compounds, ensuring consistency, clarity, and ease of communication in the scientific community. It helps in precisely describing the structure of compounds, which is crucial for research, teaching, and regulatory purposes.
How do I remember IUPAC naming rules?

+
Practice is the best method. Use mnemonics or visual aids to remember the priority of functional groups, the order of substituents, and the logical steps involved in naming compounds. Regularly review and challenge yourself with new and increasingly complex molecules.
What if I encounter a compound with a functional group I haven’t seen before?
+Research the functional group. Look up its suffix or prefix, understand its priority, and how it integrates with the rest of the IUPAC rules. Learning new groups and their nomenclature is part of the chemistry education journey.