5 Essential Facts About Isotopes, Ions, and Atoms

Understanding the fundamental concepts of isotopes, ions, and atoms is crucial for grasping the complexities of chemistry, physics, and material science. These terms are interconnected and form the bedrock of modern scientific understanding. Let's delve into what makes them unique and how they contribute to our knowledge of the universe.
Understanding Atoms

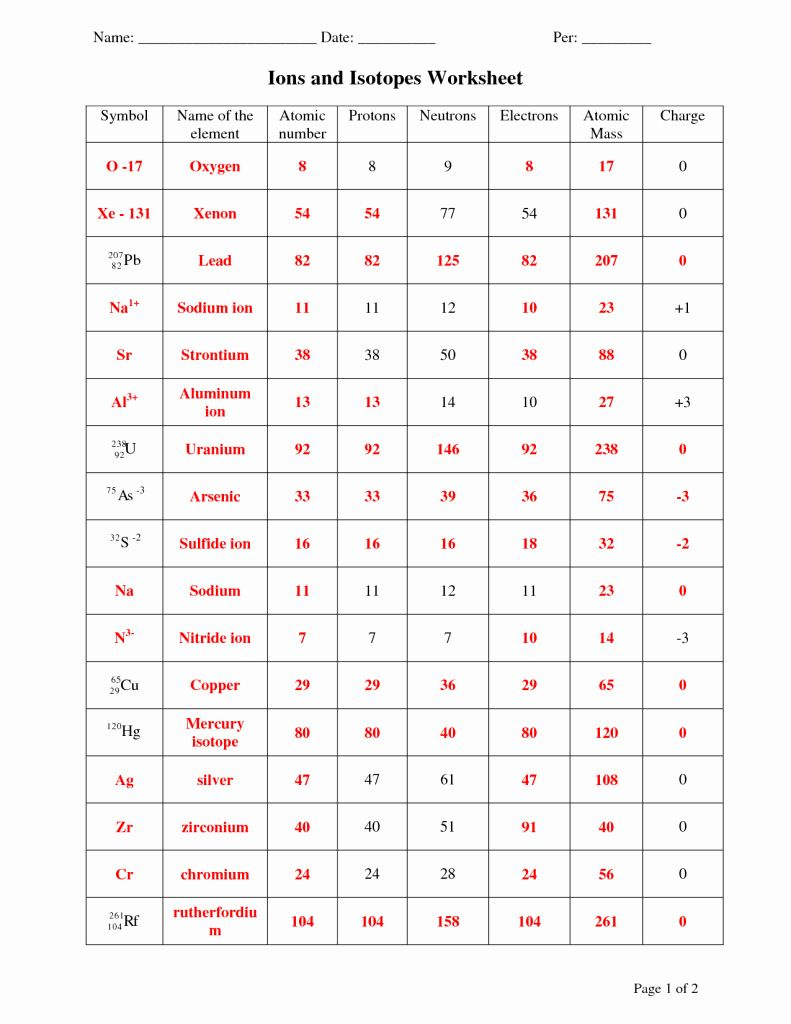
Atoms are the smallest unit of matter that retains the chemical properties of an element. They are the building blocks of all substances, from the simplest gases to the most complex compounds. Here's what you need to know about atoms:
- Composition: An atom consists of a nucleus, made of protons and neutrons, surrounded by a cloud of electrons.
- Protons and Neutrons: Protons have a positive charge, while neutrons are neutral. The number of protons determines the element's atomic number.
- Electrons: Electrons orbit the nucleus in shells or energy levels, and they have a negative charge equal to that of a proton. Their movement around the nucleus explains the electron cloud model.
- Mass: The mass of an atom is nearly the same as the sum of the protons and neutrons in the nucleus, since electrons contribute very little to the overall mass.

Isotopes and Their Significance

Isotopes are atoms of the same element that have a different number of neutrons. Here are key points about isotopes:
- Nuclear Configuration: Isotopes have identical atomic numbers (number of protons) but different mass numbers due to variations in neutron count.
- Properties: Chemically, isotopes behave similarly because their chemical properties are defined by the number of electrons, not neutrons. However, their physical properties can differ due to the change in mass.
- Applications: Isotopes are used in various applications:
- Radioactive isotopes can be used in medicine for diagnostics and treatment, like Carbon-14 in radiocarbon dating.
- Stable isotopes are useful in geochemistry and as tracers in biological and environmental studies.
- Naturally Occurring and Synthetic: Most elements have at least one isotope that occurs naturally. However, some isotopes are artificially created in nuclear reactors or particle accelerators.
| Element | Isotope | Neutron Count | Uses |
|---|---|---|---|
| Carbon | C-12 | 6 | Standard in mass spectrometry |
| Carbon | C-14 | 8 | Radiocarbon dating, biological tracer |
| Uranium | U-235 | 143 | Nuclear fuel |
⚛️ Note: Isotopes of elements can have vastly different half-lives, which can range from fractions of a second to millions of years.
Exploring Ions

Ions are atoms or molecules that have lost or gained one or more electrons, thus acquiring an electric charge. Here's how ions fit into the broader picture:
- Cations and Anions:
- Cations are positively charged ions (lost electrons).
- Anions are negatively charged ions (gained electrons).
- Formation: Ions form when atoms gain or lose electrons to achieve a stable electron configuration, typically that of a noble gas.
- Importance: Ions play crucial roles in:
- Chemistry, where they can participate in ionic bonding.
- Biology, where ions like sodium (Na+) and potassium (K+) are essential for cell function.
- Physics, with applications in spectroscopy and ion propulsion systems in spacecraft.

⚡️ Note: Ions significantly influence the properties of materials. For example, sodium chloride (NaCl) behaves differently when its constituent ions are free in solution or bonded in a lattice structure.
Interplay between Isotopes and Ions

The relationship between isotopes and ions is fascinating:
- Mass Spectrometry: Mass spectrometry separates ions based on their mass-to-charge ratio, often distinguishing isotopes.
- Nuclear Magnetic Resonance (NMR): This technique can measure the behavior of atomic nuclei, which differs among isotopes.
- Isotopic Substitution: In certain experiments, isotopic substitution can provide insight into reaction mechanisms by altering the kinetics or dynamics of ions.
Applications and Everyday Life

While isotopes and ions might seem like abstract concepts, they have practical implications:
- Food Safety:
- Isotopes like Strontium-90 are monitored in foods to ensure no radioactive contamination.
- Energy Production:
- Uranium-235 and Uranium-238 isotopes are used in nuclear reactors to produce electricity.
- Medical Imaging:
- Technetium-99m, an isotope, is widely used in medical diagnostics for imaging the body’s internal organs.

🌿 Note: The isotopic composition of water can be used to trace water movement in the environment and to understand climatic changes.
In summary, understanding isotopes, ions, and atoms not only unlocks the beauty of the microcosmic world but also empowers us to harness natural phenomena for practical applications. They are the stepping stones for countless innovations in science and technology, from the devices we use daily to the medical treatments that save lives. Through these concepts, we better comprehend the unity and diversity within matter, how atoms come together to form the universe, and the intricate dance of particles that shapes our existence.
What is the difference between isotopes and ions?

+
Isotopes are different versions of an element with the same number of protons but different numbers of neutrons, affecting the atomic mass but not the chemical behavior. Ions are atoms or molecules that have lost or gained one or more electrons, resulting in a net electrical charge that changes their reactivity and interaction with other substances.
How do isotopes contribute to the study of chemistry?

+
Isotopes are pivotal in chemistry for several reasons: they provide a tool for tracing and studying reactions, are used in mass spectrometry to identify and analyze substances, and their behavior can give insights into the structure and dynamics of molecules.
Why are ions important for biological processes?

+
Ions are essential for life because they participate in various biological processes. For instance, sodium and potassium ions maintain nerve impulse transmission, calcium ions are crucial for muscle contraction and bone formation, and hydrogen ions (H+) are involved in pH regulation.