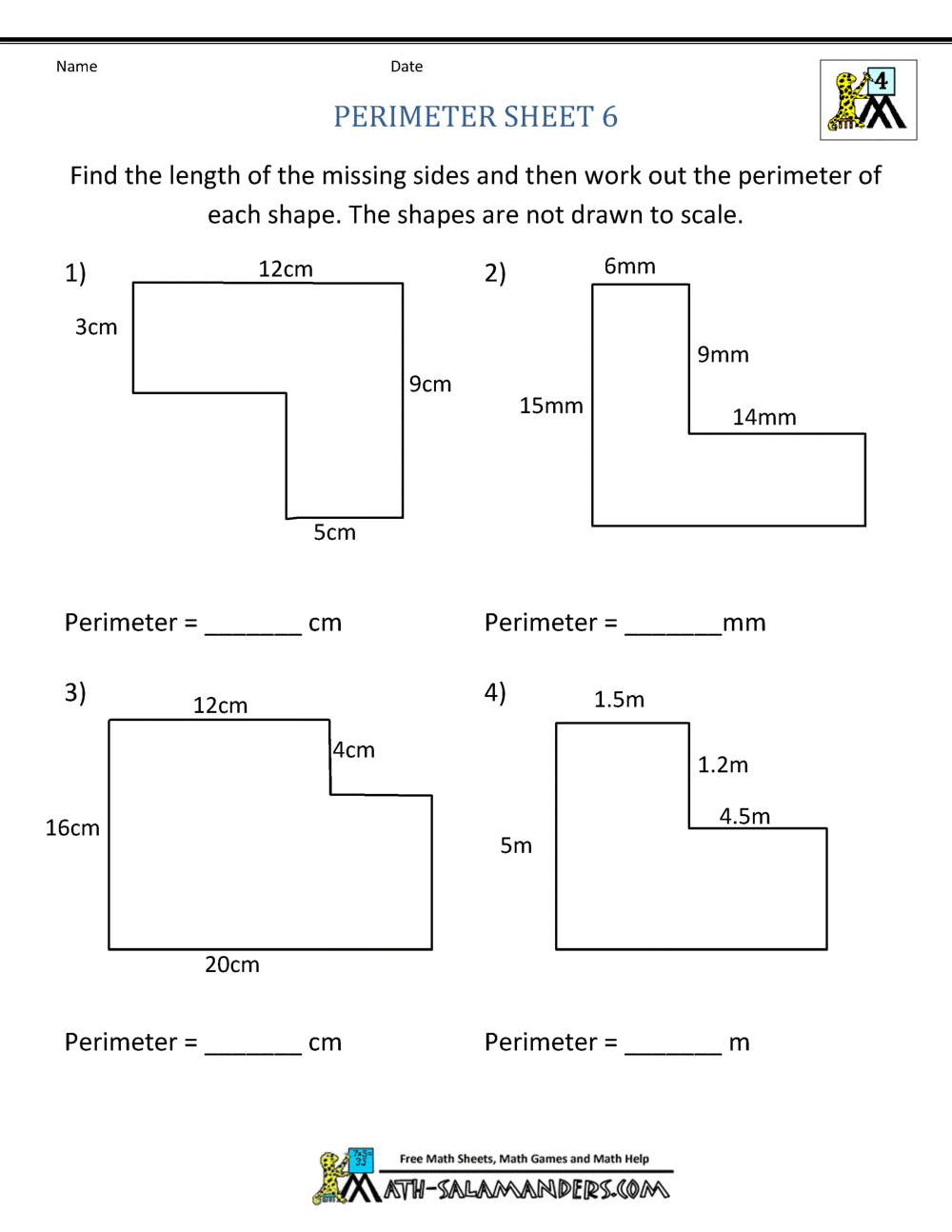
5 Tips for Understanding Isotopes and Ions
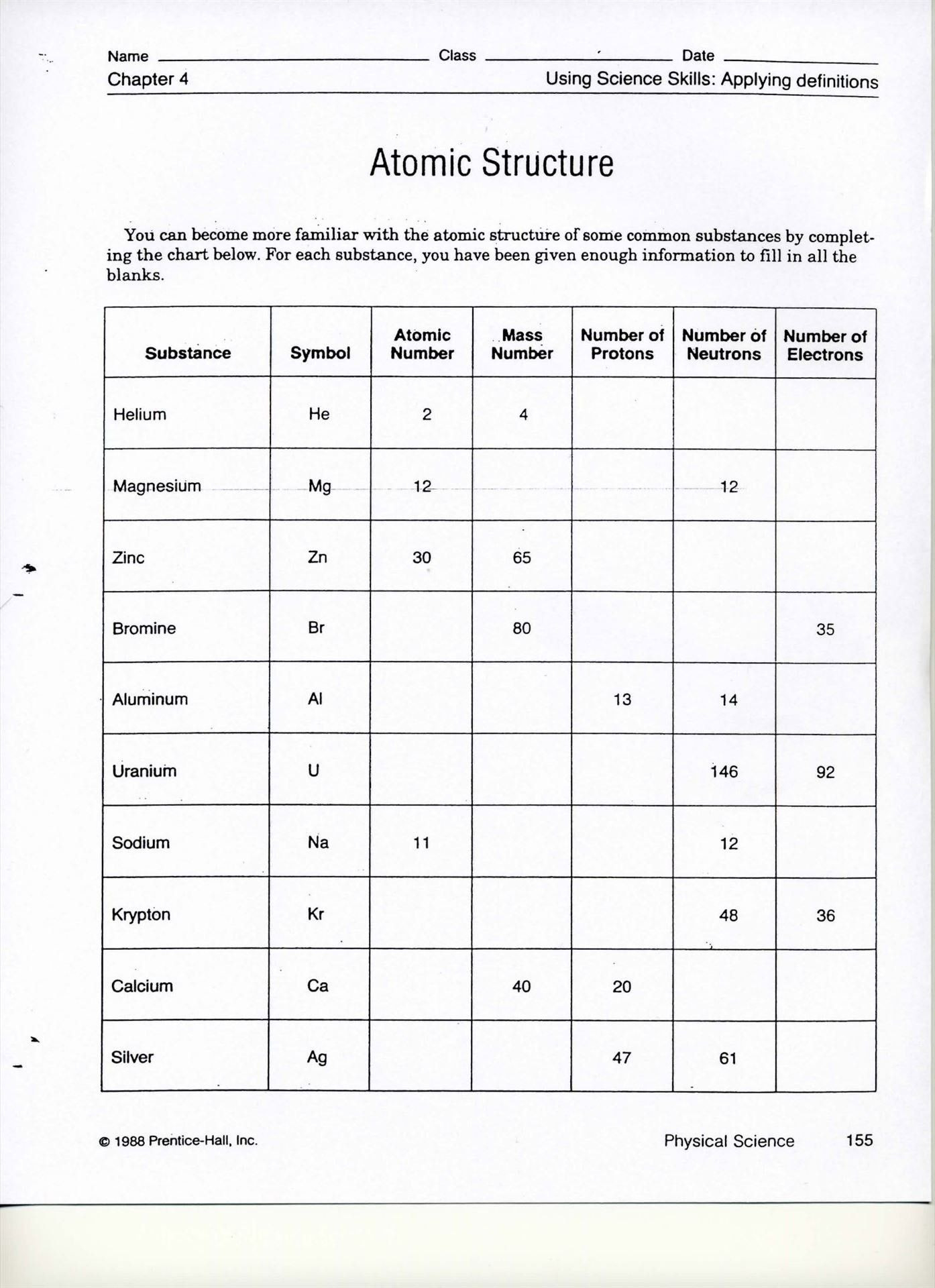
Getting Started with the Basics

Isotopes and ions are fundamental concepts in chemistry, often introducing students to the intricate world of atoms and their behaviors. But what exactly do these terms mean, and how do they relate to each other? This blog post will guide you through five essential tips to help you understand isotopes and ions, making these concepts more approachable and digestible.
1. Know Your Atomic Structure

To understand isotopes and ions, you first need to grasp the basic structure of an atom:
- Protons: Positively charged particles found in the nucleus, which define an element’s atomic number.
- Neutrons: Neutral particles also located in the nucleus, which can vary without changing the element’s identity.
- Electrons: Negatively charged particles that orbit the nucleus and determine the atom’s chemical properties.
2. Understanding Isotopes

Isotopes are versions of the same element that differ in the number of neutrons in their nuclei:
- Definition: Atoms of the same element (same number of protons) but with different mass numbers due to a variation in neutron count.
- Example: Carbon-12, Carbon-13, and Carbon-14 are all isotopes of carbon.
Here's a simple table to illustrate how isotopes differ:
| Isotope | Protons | Neutrons | Mass Number |
|---|---|---|---|
| Carbon-12 | 6 | 6 | 12 |
| Carbon-13 | 6 | 7 | 13 |
| Carbon-14 | 6 | 8 | 14 |

⚛️ Note: Isotopes have the same chemical properties but can differ in their physical properties due to the difference in mass.
3. Delving into Ions

Ions are atoms or molecules that have gained or lost electrons, thus possessing a net electric charge:
- Cations: Positively charged ions that have lost one or more electrons.
- Anions: Negatively charged ions that have gained one or more electrons.
For instance, when sodium (Na) loses an electron, it becomes Na+, a cation. Conversely, when chlorine (Cl) gains an electron, it becomes Cl-, an anion.
4. The Relationship between Isotopes and Ions

Isotopes and ions are distinct from each other:
- Isotopes: Alteration in the nucleus (neutron count) doesn’t affect electron shells or charge.
- Ions: Change in electron configuration leading to a charge, while the nucleus remains unchanged.
This distinction is crucial as it shows how atoms can exhibit multiple identities:
⚛️ Note: Isotopes are the same element with different mass numbers, whereas ions are different species of elements with a net charge.
5. Practical Applications

Both isotopes and ions have real-world applications:
- Isotopes: Used in radiometric dating (e.g., Carbon-14), nuclear energy (e.g., Uranium-235), and medical imaging (e.g., Technetium-99m).
- Ions: Essential in biological systems (e.g., Na+, K+ for nerve impulses), ion exchange in water purification, and as electrolytes in batteries.
As we conclude our exploration into isotopes and ions, it's important to appreciate how these fundamental concepts are not only intriguing but also integral to understanding the behavior of matter at the atomic level. Isotopes and ions provide insights into how elements interact, how matter changes, and how we can harness these properties for various applications. They represent the dynamic nature of atoms and their ability to form diverse compounds, affecting everything from the environment to our health.
What is the main difference between an isotope and an ion?

+
Isotopes differ in the number of neutrons, while ions differ in the number of electrons, giving them a net charge.
Can an isotope also be an ion?

+
Yes, an isotope can also be an ion. For example, Carbon-14 can lose or gain electrons to become an ion while maintaining its isotopic identity.
How do isotopes affect the mass of an element?

+
Isotopes have different masses due to varying numbers of neutrons, which affects the element’s average atomic mass reported on the periodic table.
Why are ions important in biological systems?

+
Ions like sodium (Na+) and potassium (K+) are crucial for nerve impulses, muscle contraction, and maintaining osmotic balance in cells.
What is the role of isotopes in age determination?

+
Isotopes like Carbon-14 decay at a known rate, allowing scientists to date organic materials by measuring the remaining amount of this isotope, a process known as radiometric dating.