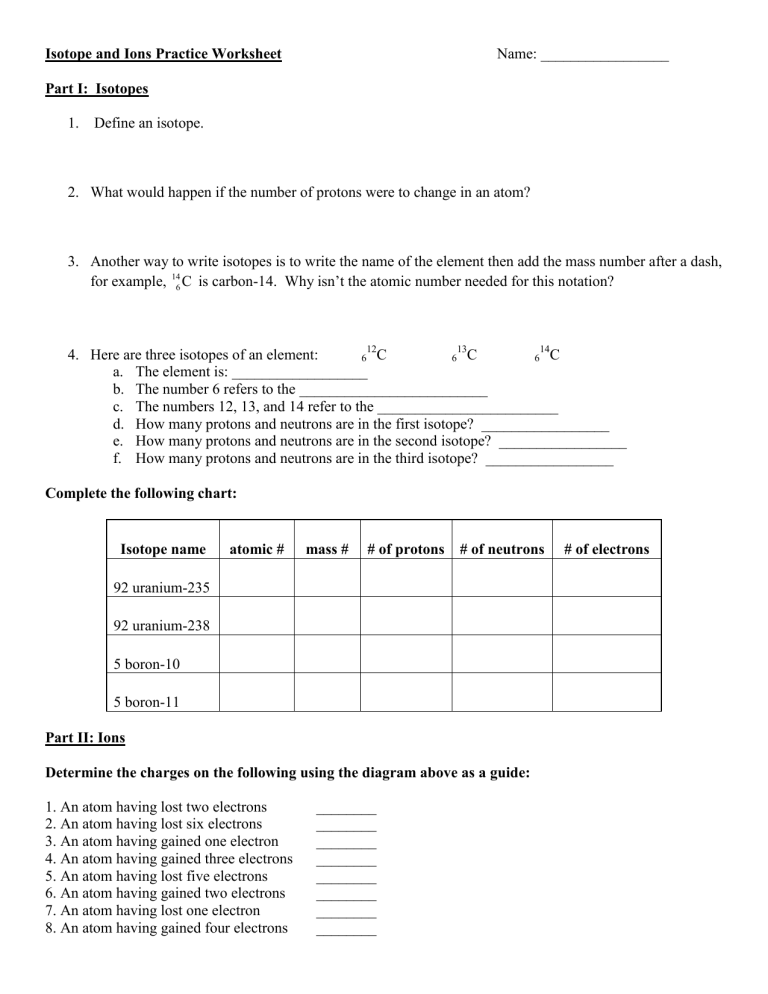
Master Isotopes and Ions: Engaging Practice Worksheet
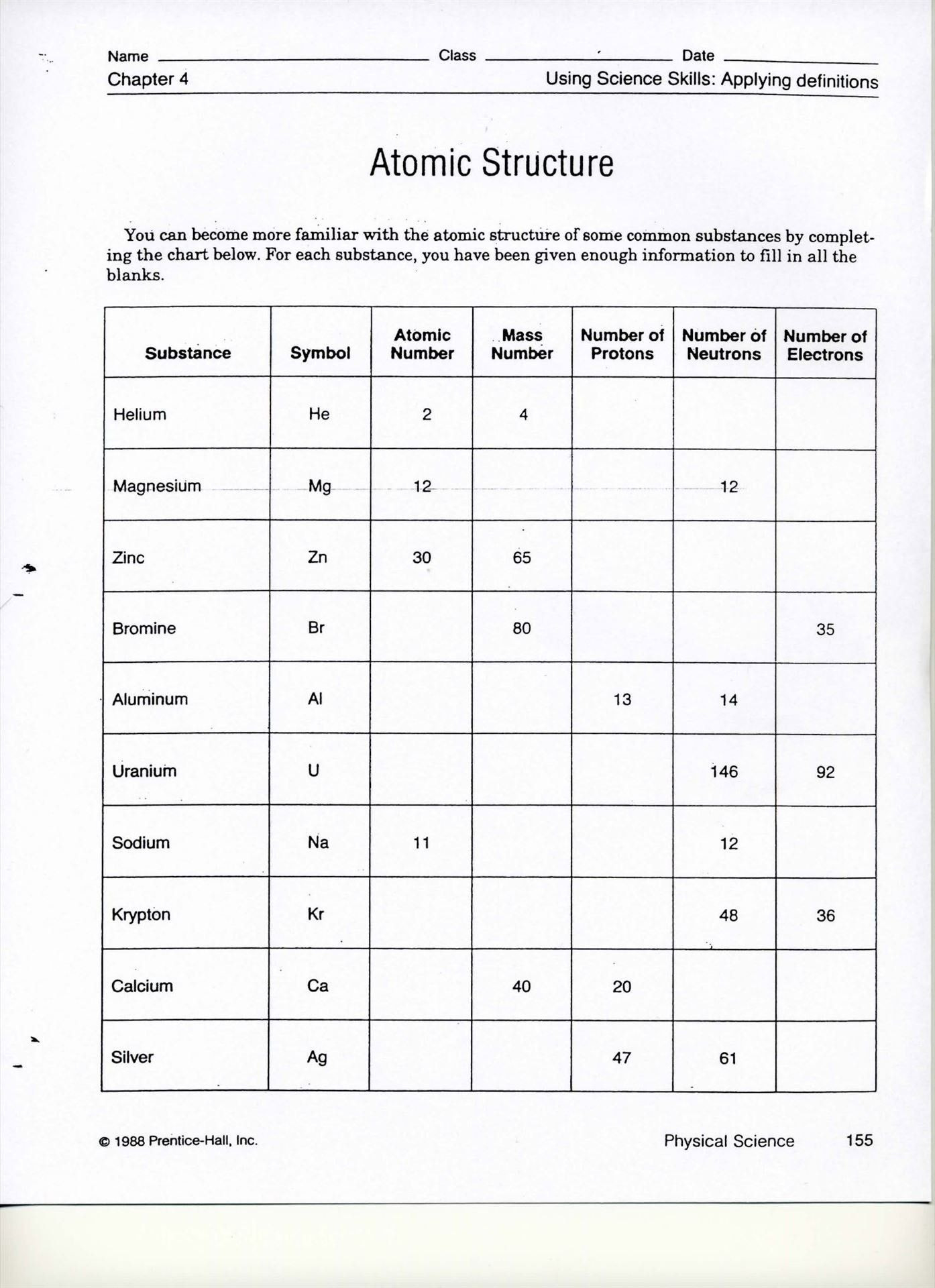
Understanding the world of chemistry involves a dive into its fundamental building blocks—atoms. Through natural variations and interactive processes, atoms can become more intriguing forms known as isotopes and ions. For students, mastering these concepts is not just an academic pursuit but a means to unravel the complexities of matter at a microscopic level. This blog post offers an engaging worksheet to explore isotopes and ions, helping learners grasp the elemental particles, their charges, and their significance in chemistry.
What Are Isotopes?

Isotopes are versions of an element with the same number of protons but different numbers of neutrons. This variance leads to differences in atomic mass without changing the element’s chemical properties significantly.
- Number of Protons: Constant for each element.
- Number of Neutrons: Can vary, leading to isotopes.
Practice with Isotopes
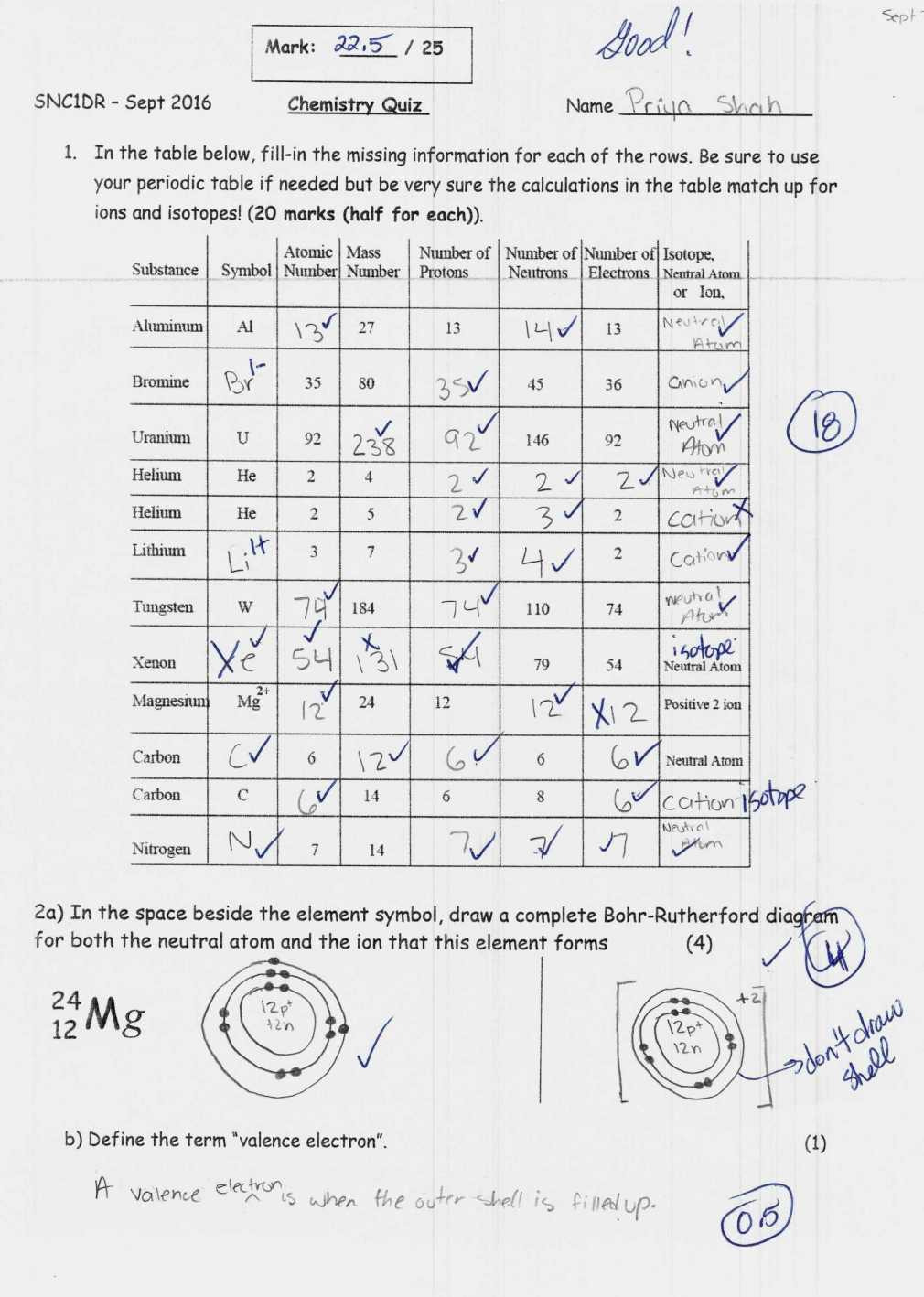
Let’s engage with a couple of exercises:
| Element | Protons | Neutrons | Notation | Atomic Mass |
|---|---|---|---|---|
| Hydrogen | 1 | 0 | ₁H¹ | 1.007825 u |
| Carbon | 6 | 6 | ₆C¹² | 12 u |
| Oxygen | 8 | 10 | ₈O¹⁸ | 18.0009 u |

📝 Note: The atomic mass of isotopes is calculated as the average of all naturally occurring isotopes, factoring in their abundance.
Ions: Charged Particles
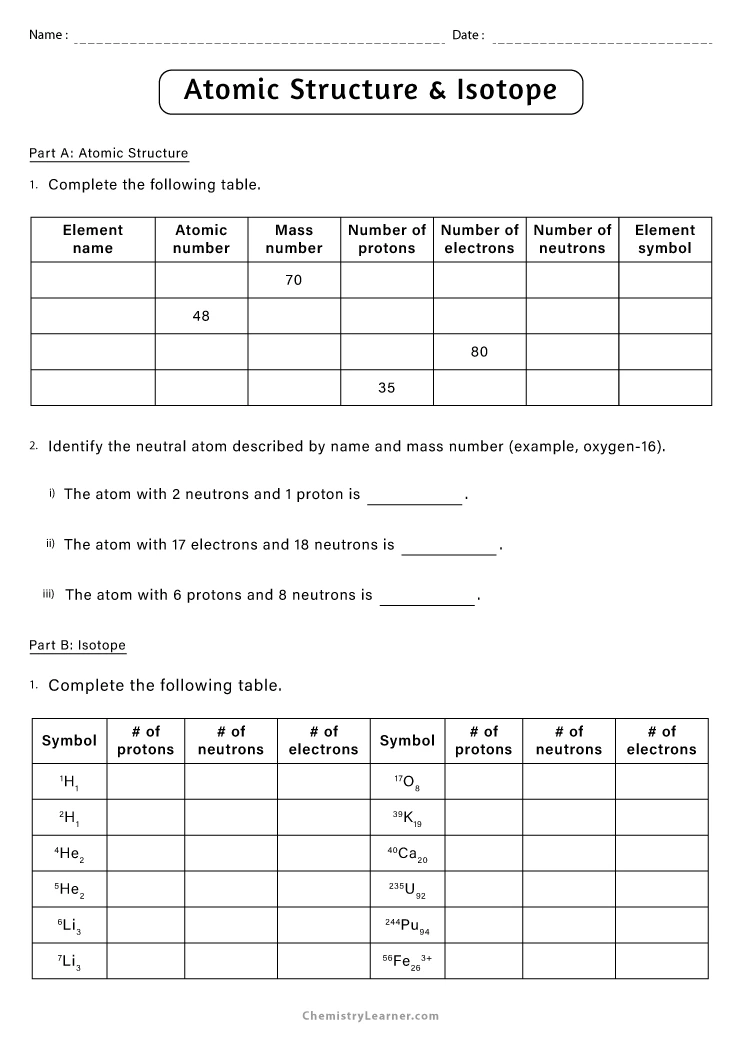
An ion is an atom or molecule with an unequal number of electrons and protons, leading to a net electrical charge. Here are the key distinctions:
- Cations: Positively charged ions; lose electrons.
- Anions: Negatively charged ions; gain electrons.
Practice with Ions

Consider these scenarios:
- If a sodium atom (Na) loses an electron, what charge does it acquire?
- If an oxygen atom (O) gains two electrons, what is the resulting charge?
Answers:
- Sodium atom loses an electron to become Na⁺ with a charge of +1.
- Oxygen atom gains two electrons to become O²⁻ with a charge of -2.
Integrating Isotopes and Ions

The real-world application of isotopes and ions is vast, from nuclear energy to medical diagnostics:
- Nuclear Medicine: Radioactive isotopes like Technetium-99m help visualize internal structures.
- Electrolyte Balance: Ions like Na⁺ and Cl⁻ maintain bodily functions and hydration.
Key Points Recap
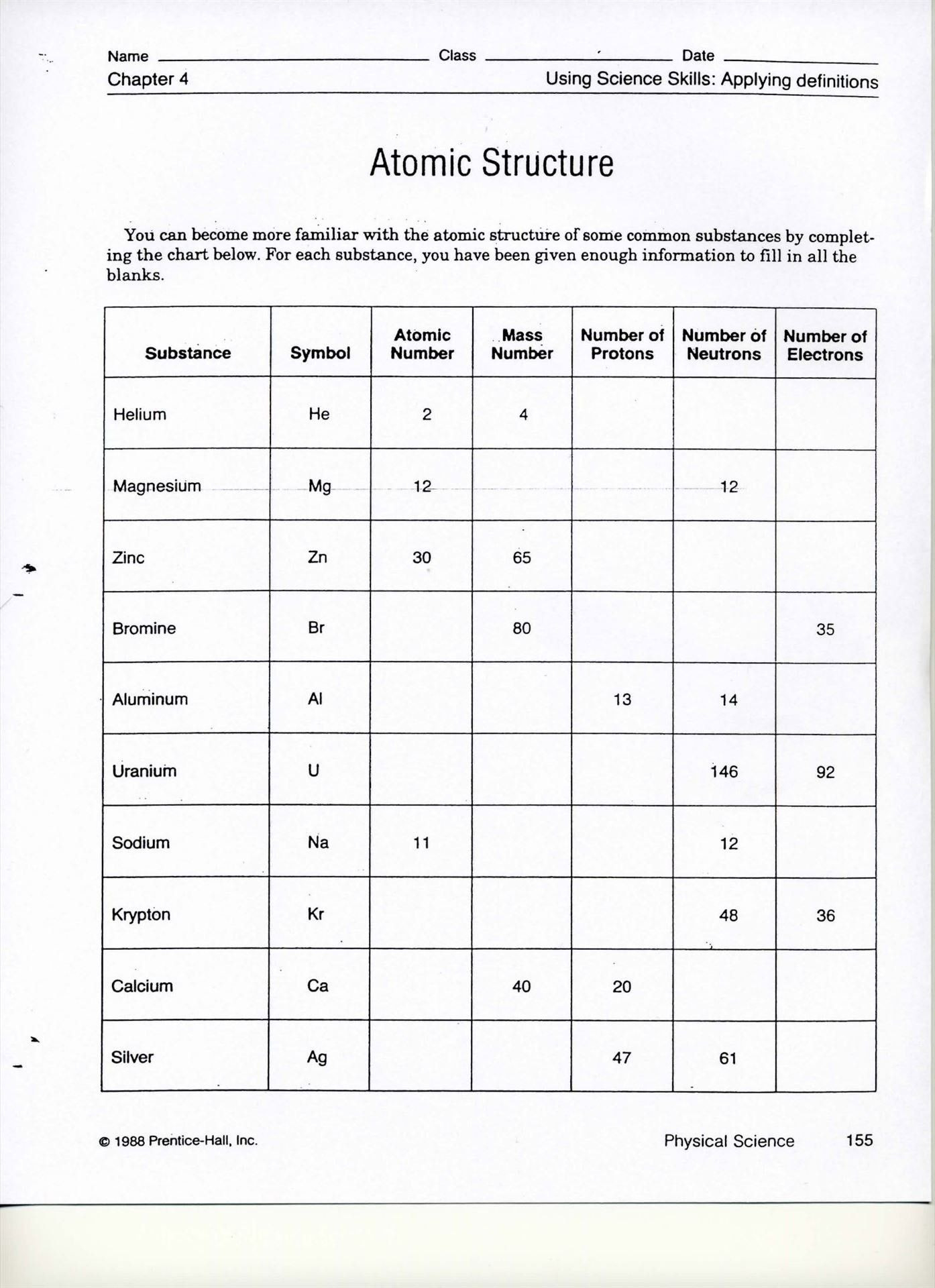
In our journey through the world of chemistry, we've:
- Explored what isotopes are, their unique characteristics.
- Learned about ions, their formation, and properties.
- Practiced identifying isotopes and understanding ions through exercises.
In closing, isotopes and ions play a critical role in the nature of matter, atomic structures, and countless applications in our daily lives. Their study enriches our understanding and offers insights into how the universe functions at its most elemental level. The worksheet provided here can serve as an excellent tool for students to practice, understand, and appreciate these fascinating aspects of chemistry.
What makes isotopes different from each other?
+
Isotopes differ due to their varying numbers of neutrons, which affects their atomic mass but not their chemical behavior.
Why do ions carry a charge?

+
Ions carry a charge because they have an unequal number of protons and electrons. Losing electrons results in a positive charge (cations), and gaining electrons leads to a negative charge (anions).
How can I identify an isotope?

+
An isotope is identified by its element symbol followed by its mass number (sum of protons and neutrons). For example, Deuterium is written as ₂H², indicating it has 2 neutrons.