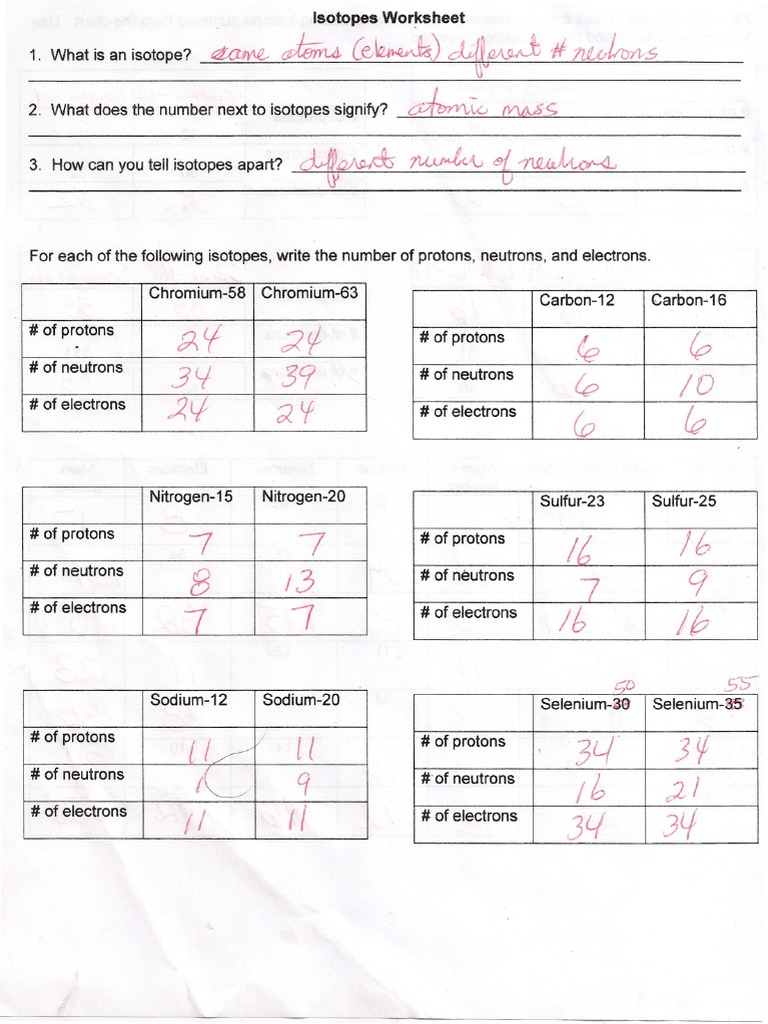
5 Essential Isotope Worksheet Answers You Need

Isotopes are variants of a particular chemical element that differ in neutron number, consequently impacting the nucleus's mass. The study of isotopes forms a cornerstone in physics, chemistry, and even biology, with applications stretching from nuclear science to medical diagnostics. Today, we'll delve into the essentials of isotopes, offering answers to the five most commonly posed questions in isotope worksheets that you are likely to encounter as a student or hobbyist in this fascinating field.
1. Defining Isotopes: What Are They?

At the core of understanding isotopes is defining what they are. An isotope of an element retains the same number of protons (atomic number), but has a different number of neutrons compared to other isotopes of that same element.
- The atomic number defines the element.
- Neutron count changes the mass number.
- The mass number is the sum of protons and neutrons in the nucleus.
Isotopes can be stable or radioactive, meaning they do not change over time or will decay into other elements, releasing particles and energy. For instance, Hydrogen has three isotopes:
| Isotope | Symbol | Protons | Neutrons | Stability |
|---|---|---|---|---|
| Protium | 1H | 1 | 0 | Stable |
| Deuterium | 2H or D | 1 | 1 | Stable |
| Tritium | 3H or T | 1 | 2 | Radioactive |

⚠️ Note: While most isotopes have practical uses, some can be dangerous due to their radioactivity. Proper handling and understanding are necessary.
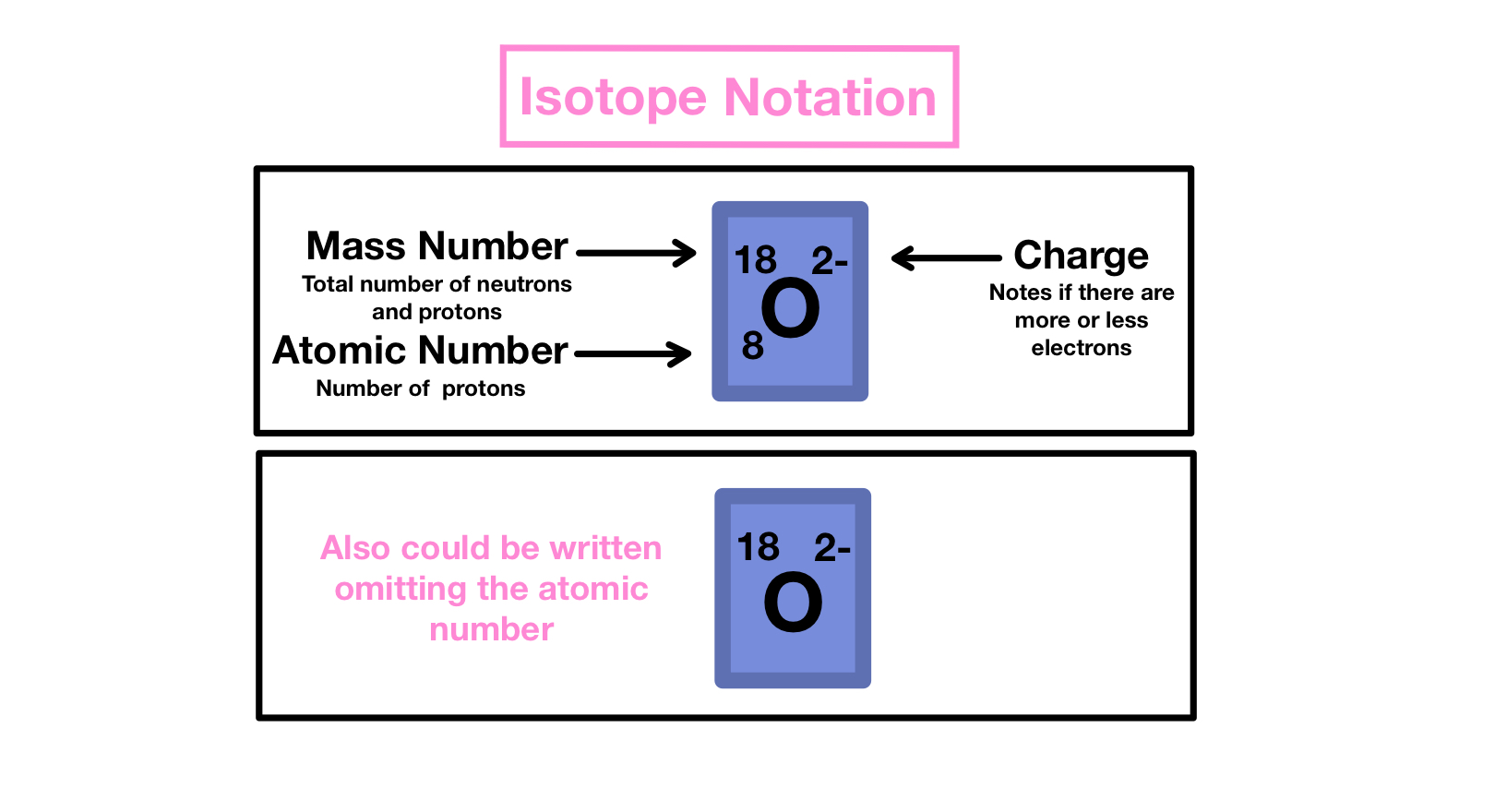
2. Writing Isotope Symbols and Calculating Atomic Mass

To represent isotopes, we use isotopic notation. Here's how to write isotope symbols:
- The element symbol is used.
- The mass number is written as a superscript to the left of the element symbol.
- The atomic number is written as a subscript to the left of the element symbol.
For example, 12C denotes a Carbon atom with an atomic mass of 12, consisting of 6 protons and 6 neutrons. The atomic mass of an element reflects the average mass of all naturally occurring isotopes, weighted by their natural abundance.
3. Isotopic Abundance: Why It Matters
Knowing the abundance of different isotopes of an element in nature is crucial:
- It informs the average atomic mass of elements, which is essential in chemistry and physics calculations.
- Abundances help in geochemical studies for dating rocks or biological remains.
- It provides insights into nuclear processes in stars and how elements are synthesized.
For example, Chlorine has two major isotopes: Cl-35 (75.77%) and Cl-37 (24.23%). Using their masses (34.97 u and 36.97 u, respectively), the average atomic mass is calculated:
\[ \text{Average Atomic Mass} = (34.97 \times 0.7577) + (36.97 \times 0.2423) = 35.45 \, u\]
💡 Note: This calculation demonstrates how relative abundance impacts the average atomic mass, an essential concept in understanding the periodic table and isotopic characteristics.
4. Uses of Radioactive Isotopes

The field of nuclear medicine relies heavily on radioactive isotopes:
- Medical Imaging: Isotopes like Tc-99m are used in scans to diagnose and monitor conditions.
- Cancer Treatment: Radiation therapy often uses isotopes like Co-60 to target cancer cells.
- Food Preservation: Gamma irradiation with isotopes like Co-60 or Cs-137 can sterilize food, extending shelf life and safety.
- Power Generation: Nuclear power plants harness the energy from nuclear reactions of isotopes such as U-235 or Pu-239.
The uses extend to scientific research, industrial applications, and archaeological dating, where isotopic abundance can inform the age of artifacts or ancient remains.
5. The Role of Isotopes in Understanding the Universe

Isotopes play a critical role in unraveling the mysteries of the cosmos:
- Stellar Evolution: By studying isotopic abundances, scientists can infer the life cycles of stars and how elements are formed.
- Cosmic Ray Analysis: Isotopes like Be-10 are produced when cosmic rays interact with Earth's atmosphere, providing a snapshot of space's influence.
- Climate Science: Isotopic ratios in ice cores or tree rings help reconstruct past climate conditions.
The final insights from our exploration of isotopes reveal that they are not just curiosities; they are vital to our understanding of our planet, its history, and the universe's evolution. Through the study of isotopes, we gain knowledge about elemental stability, nuclear forces, and even the processes that might have seeded life itself.
Understanding isotopes is not merely a scientific pursuit; it's an educational journey that connects various fields of study, enhancing our appreciation for the complexity of nature. From the inner workings of atomic nuclei to the expansive scale of galaxy formations, isotopes provide key data points that help us piece together the grand cosmic puzzle.
What are isotopes and why are they important in science?
+
Isotopes are atoms of the same element with different numbers of neutrons, which results in different mass numbers. Their importance in science lies in their applications in medicine, geology, dating methods, and understanding nuclear processes, offering a wide range of information from atomic to cosmic scales.
How is isotopic abundance calculated?

+
Isotopic abundance is calculated by determining the percentage of each isotope present in a sample of the element. These percentages are then used to calculate the average atomic mass of the element, considering the mass of each isotope and its relative abundance.
What are stable and unstable isotopes?

+
Stable isotopes do not undergo radioactive decay and remain consistent over time. Unstable, or radioactive isotopes, decay over time by emitting particles or radiation to reach a more stable state, which can release energy or transform into another element or isotope.