Unlock Isotope Secrets: Practice Worksheet Answers Revealed

Exploring isotopes might seem like a daunting task due to the sheer complexity of atomic theory, but it's an incredibly fascinating part of chemistry that opens up a world of understanding about how elements behave and interact. In this blog, we'll unlock the secrets behind isotopes by focusing on an educational resource known as a practice worksheet. By working through the answers, we will deepen our understanding of isotopes and make learning this concept both accessible and enjoyable for students and curious minds alike.
Understanding Isotopes

Before we delve into the worksheet answers, let’s grasp what isotopes are:
- Isotopes: Atoms of the same element with the same number of protons but different numbers of neutrons.
- Notation: Isotopes are often written in a form where the atomic number (number of protons) is displayed below the chemical symbol, and the mass number (sum of protons and neutrons) is above it, e.g., 12C 6.
- Atomic Weight: An average of the mass numbers of all naturally occurring isotopes of an element, factoring in their abundance.

The Practice Worksheet: Key Sections

Here’s what to expect in a typical isotopes practice worksheet:
- Identification of Isotopes
- Calculating Atomic Mass
- Determining Isotopic Abundance
- Real-Life Applications
1. Identification of Isotopes
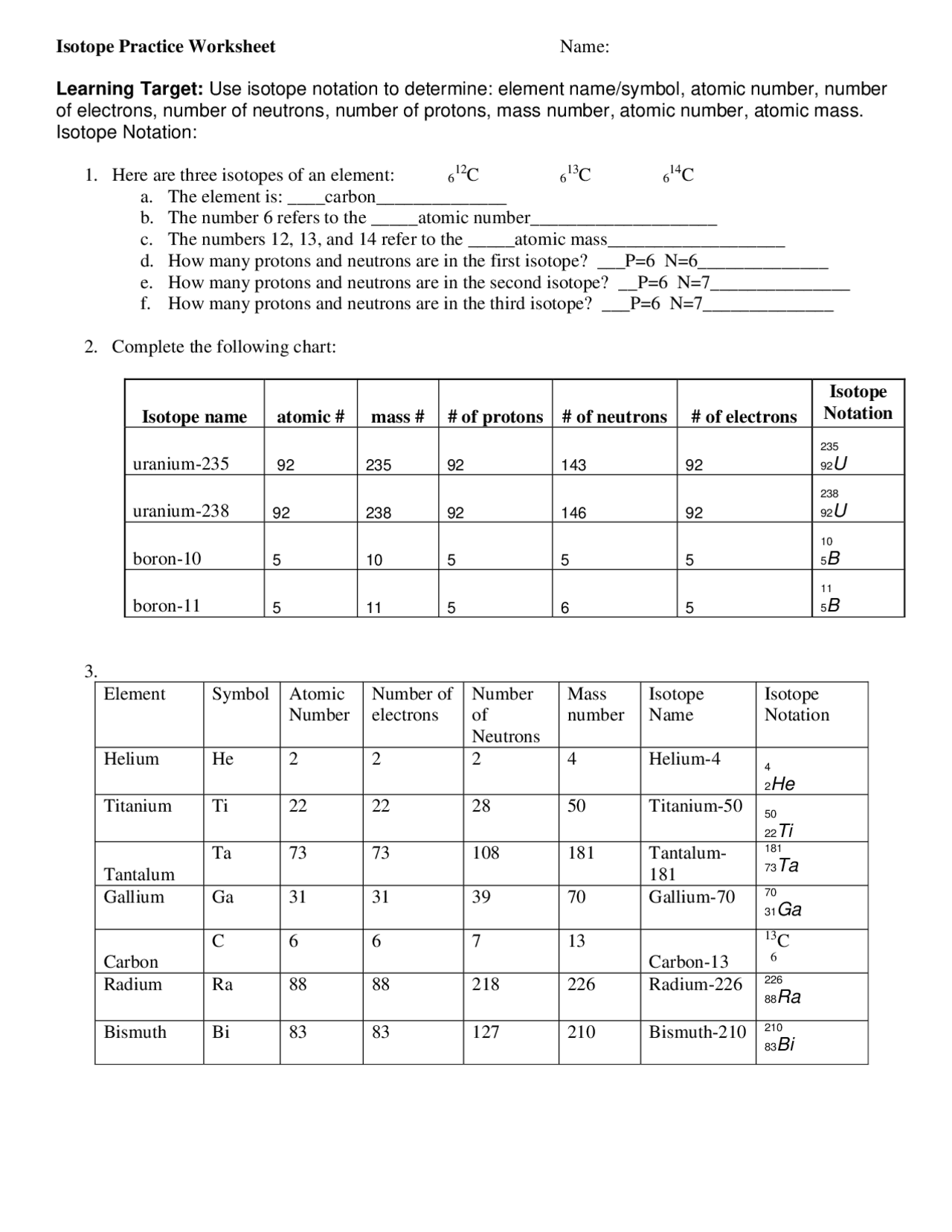
Here, you identify isotopes based on given data:
| Element | Protons | Neutrons | Isotope Notation |
|---|---|---|---|
| Carbon | 6 | 6 | 12C 6 |
| Carbon | 6 | 7 | 13C 6 |
| Uranium | 92 | 146 | 238U 92 |

By working through these examples, we understand how isotopes are noted and the significance of their mass number.
2. Calculating Atomic Mass

Calculating atomic mass involves:
- Multiplying the mass of each isotope by its abundance percentage.
- Summing these products for all isotopes of an element.
Example:
Carbon has two stable isotopes:
- 12C has a relative abundance of 98.93%
- 13C has a relative abundance of 1.07%
Atomic mass of Carbon = (0.9893 × 12) + (0.0107 × 13) ≈ 12.01 amu
3. Determining Isotopic Abundance
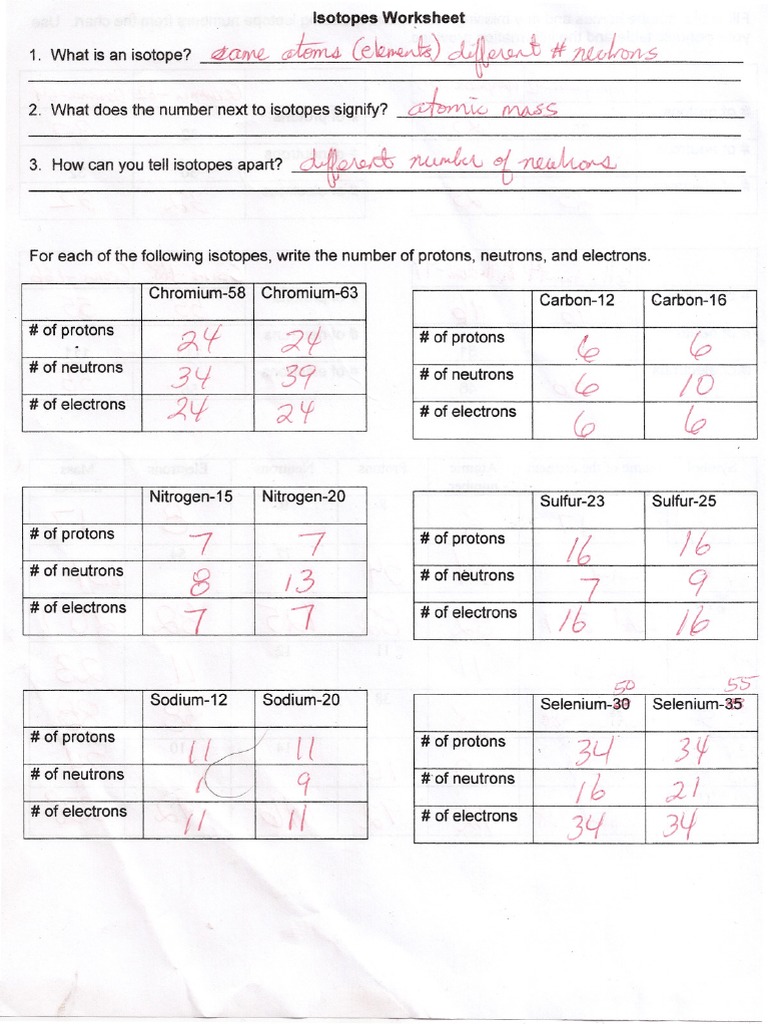
If the atomic mass is known and you are given isotopic masses, you can determine isotopic abundance:
Example:
- Silicon has an atomic mass of 28.0855 amu.
- There are three stable isotopes: 28Si, 29Si, and 30Si.
- Let x be the abundance of 28Si, and y be the abundance of 29Si.
Using a system of equations:
x + y + (1 - x - y) = 1
(0.28 x) + (0.29 y) + [30 × (1 - x - y)] = 28.0855
This equation helps us determine isotopic abundances from known atomic masses.
4. Real-Life Applications

Isotopes are not just theoretical constructs; they have practical applications:
- Medical Field: Isotopes like Tc-99m for imaging, and I-131 for thyroid treatment.
- Environmental Tracking: Carbon isotopes (14C) are used in carbon dating.
- Power Generation: Uranium-235 is used in nuclear reactors.
By providing answers to a practice worksheet, we've not only learned to handle the numbers and notation associated with isotopes but also appreciated their practical significance. This knowledge not only boosts exam performance but also enriches our understanding of the world at an atomic level.
💡 Note: Always consider the natural abundance when calculating atomic mass; most elements are a mixture of isotopes in varying proportions.
Having dissected isotope theory and put it into practice through worksheet exercises, we've covered a wealth of information that makes us ready to engage with this topic in more depth. Isotopes are not just a part of chemistry or physics; they have an interdisciplinary presence in medicine, archaeology, and environmental science, highlighting the unity of scientific inquiry. By understanding isotopes, we are equipped to ask and answer deeper questions about our universe and the natural world around us.
What is an isotope?

+
An isotope is a variant of an element that has the same number of protons (atomic number) but a different number of neutrons. This means they differ in atomic mass but have the same chemical properties.
How are isotopes written?

+
Isotopes are typically written in the form of AEZ where A is the mass number (number of protons plus neutrons), E is the chemical symbol of the element, and Z is the atomic number (number of protons).
What are isotopes used for?

+
Isotopes have numerous applications, including medical diagnostics and treatments, archaeological dating (carbon-14), nuclear power generation, and as tracers in scientific research to follow the path of substances in biological and chemical systems.
Why is it important to know isotopic abundances?
+
Understanding isotopic abundances is key to calculating the atomic weight of an element, which influences chemical reactions and behavior of elements. It’s also crucial for interpreting mass spectrometry data and understanding isotopic effects in nature.
Can isotopes change into one another?

+
Yes, isotopes can change through radioactive decay, a process where an unstable isotope emits particles or electromagnetic radiation to achieve stability. However, this transformation is not a simple conversion but involves changes in the number of protons and neutrons.