Isotope Practice Worksheet: Master Chemistry Basics Easily
In the world of chemistry, understanding isotopes and their properties is crucial for mastering the basic concepts and achieving proficiency in more complex chemical analysis. This guide offers an easy and efficient method to practice isotopes through targeted worksheets. Designed to reinforce your knowledge in isotope chemistry, this article will walk you through the practical aspects of working with isotopes, ensuring a solid foundation for your chemical studies.
What Are Isotopes?

Isotopes are variations of chemical elements that have the same atomic number but differ in the number of neutrons in their atomic nuclei. This fundamental principle underpins many aspects of nuclear chemistry:
- Chemical Similarities: Isotopes of the same element exhibit similar chemical behaviors due to the identical electron configurations.
- Physical Properties: Despite having the same chemical properties, isotopes differ in mass, which can affect physical properties like density and rate of diffusion.
- Nuclear Stability: Isotopes with specific neutron-proton ratios are stable, while others can be radioactive, undergoing decay to achieve stability.
💡 Note: Isotopes play a critical role in fields like radiometric dating, nuclear medicine, and industrial applications where knowing the isotopic composition is essential.
Basic Isotope Notation

Understanding how to read and write isotope notation is fundamental:
- Isotopes are commonly represented as AXZ, where:
- A is the mass number (protons + neutrons).
- Z is the atomic number (number of protons).
- X is the element symbol.
- For example, 14C6 denotes the carbon isotope with 6 protons and 8 neutrons.
- Alternatively, isotopes can be identified with a hyphenated number like Carbon-14.
Image Here
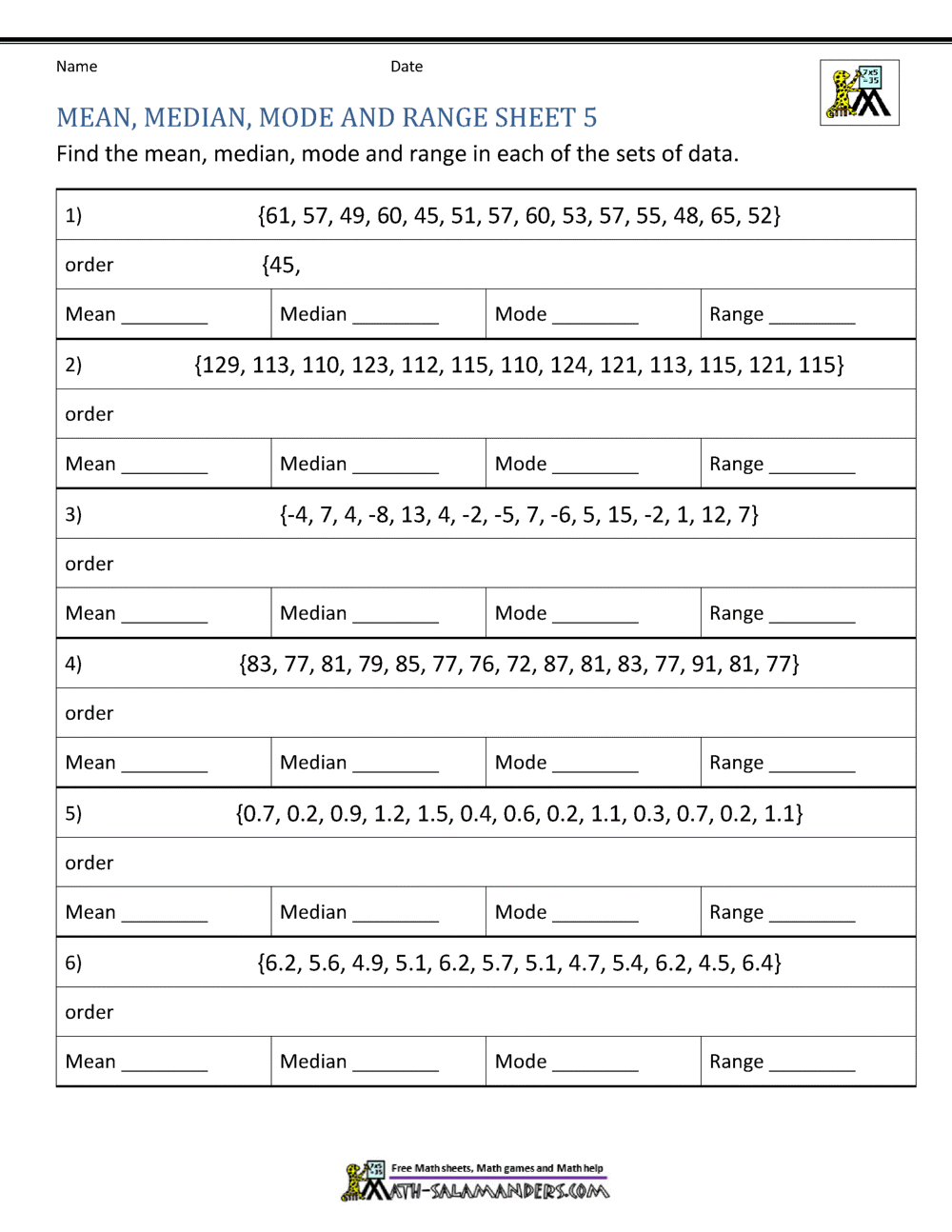
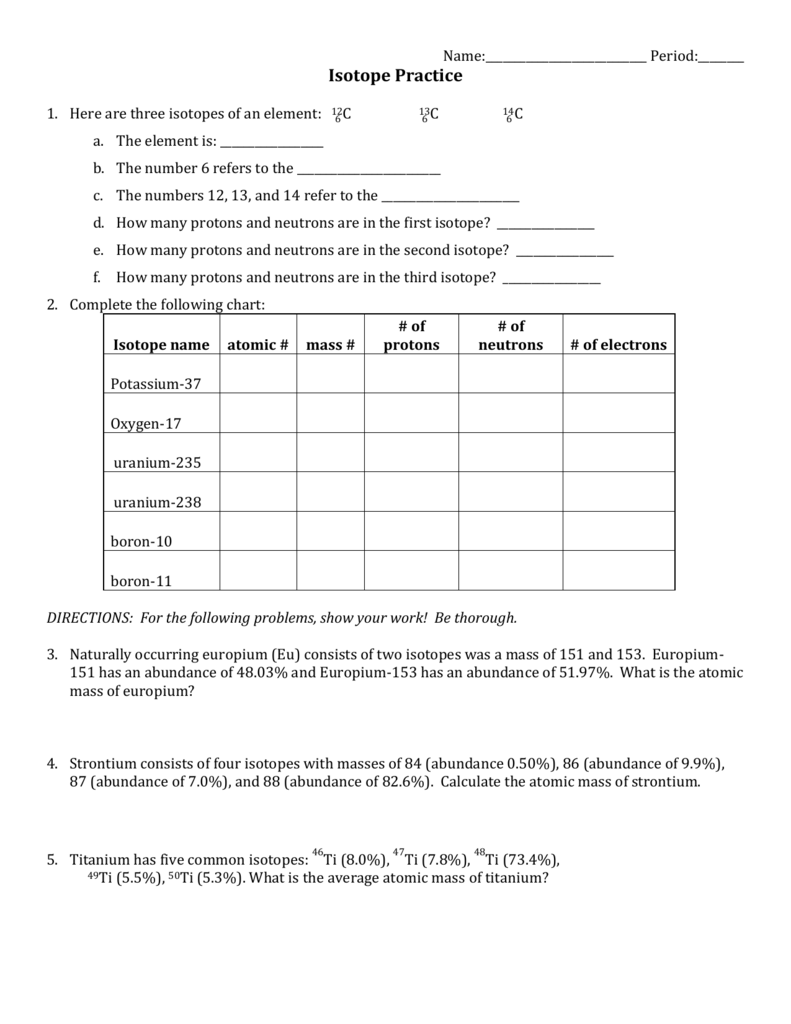
Isotope Practice Worksheet

To help solidify your understanding of isotopes, here are some practical exercises:
Calculating Neutron Number

Given the mass number (A) and atomic number (Z) of an isotope, determine the number of neutrons. Practice with these examples:
| Element | Mass Number | Atomic Number | Number of Neutrons |
|---|---|---|---|
| Oxygen | 16 | 8 | (16-8) = 8 |
| Chlorine | 37 | 17 | (37-17) = 20 |
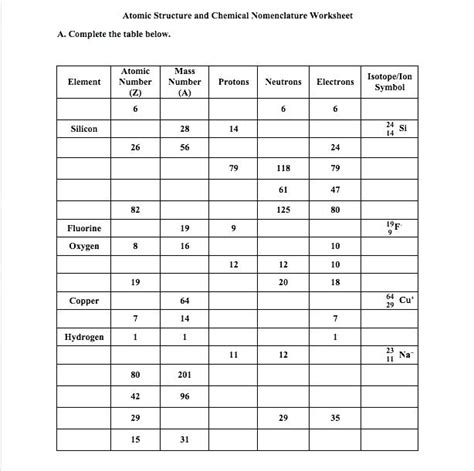
✅ Note: This practice helps in understanding how isotopic composition affects element properties.
Isotopic Composition

Practice interpreting isotopic data with these exercises:
- Find the average atomic mass of Boron using the isotopic abundances:
- 10B: 19.9%
- 11B: 80.1%
- Use the formula: Average Atomic Mass = (Isotope Mass × Abundance) + (Isotope Mass × Abundance)
- Determine the abundance of each isotope for an element with a known average atomic mass and given isotopic masses.
⚠️ Note: Always double-check your calculations, as slight errors can significantly impact the results.
Nuclear Decay

Practice predicting the product of nuclear decay for different isotopes:
- Alpha decay: loss of two protons and two neutrons (He nucleus)
- Beta-minus decay: a neutron becomes a proton with the emission of an electron
- Beta-plus decay: a proton becomes a neutron with the emission of a positron
🧪 Note: Nuclear decay equations are essential for understanding the stability and transformations of isotopes.
In mastering isotope chemistry, consistent practice with isotopes allows for a deeper comprehension of atomic structure, stability, and nuclear behavior. By completing the provided exercises, you're not only enhancing your skill in identifying isotopes and calculating their properties but also preparing for more advanced topics in nuclear chemistry. The interplay between isotopic ratios, nuclear processes, and their applications in various scientific and industrial contexts highlights the importance of a thorough understanding of isotopes.
Why do isotopes have different masses?

+
Isotopes have different masses due to the varying number of neutrons in their atomic nuclei. While all isotopes of an element have the same number of protons, the addition or subtraction of neutrons affects the overall mass of the isotope without altering its chemical identity.
How can isotopes be used in daily life?

+
Isotopes have numerous practical applications in daily life, from medical diagnostics where radioactive isotopes like Technetium-99m are used for imaging, to carbon-14 dating for archaeological purposes, and in various industrial processes where isotopic tracers help understand material flows.
What is the significance of isotopic abundance?

+
The isotopic abundance of an element influences its average atomic mass, which is crucial for many chemical calculations. Furthermore, isotopic abundance can be used in geochemistry to trace geological processes, in paleoclimatology to study past climates, and in nuclear forensics to understand the origin of nuclear material.