5 Ways to Master Isotope Notation

Understanding Isotope Notation
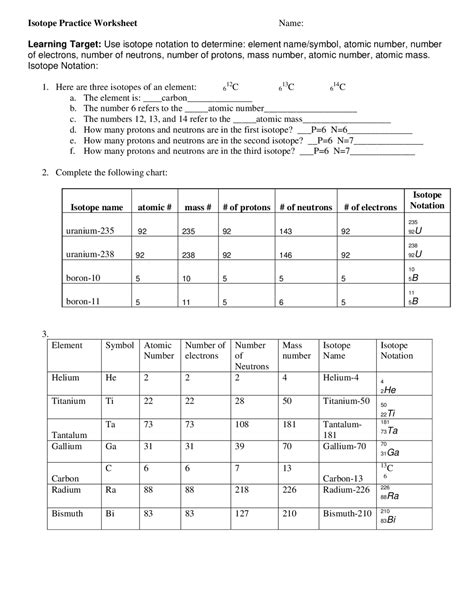
Isotope notation is a crucial concept in chemistry, enabling scientists to identify and distinguish between different isotopes of an element. An isotope is a version of an element with a varying number of neutrons in its atomic nucleus, leading to differences in mass but not in chemical properties. Mastering isotope notation is essential for chemists, physicists, and researchers to accurately describe and work with isotopes in various applications. Here are five ways to master isotope notation.
1. Learning the Basic Format
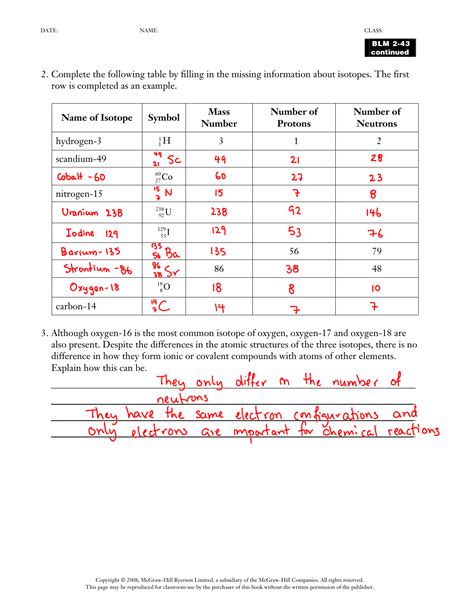
The first step in mastering isotope notation is to understand its basic format. Isotope notation is written in the form:
[^{A}_{Z}X]
Where:
- (X) is the symbol of the element (e.g., H for hydrogen, C for carbon).
- (Z) is the atomic number (number of protons) of the element.
- (A) is the mass number (total number of protons and neutrons) of the isotope.
For example, the notation for carbon-12 is:
[^{12}_{6}C]
This tells us that this isotope of carbon has 6 protons and 6 neutrons, making up a total mass number of 12.
📝 Note: The atomic number (Z) is often omitted if the element is already known, but it's crucial for clarity and accuracy in scientific communication.
2. Understanding Isotopic Variations
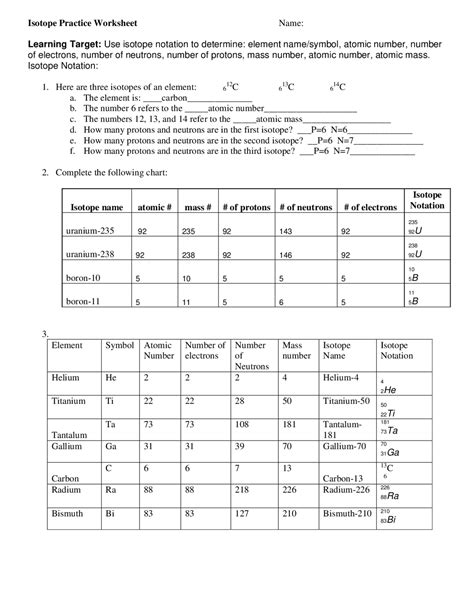
Elements can have multiple isotopes, each with a different number of neutrons. Understanding these variations is key to mastering isotope notation. Let’s consider carbon as an example:
- Carbon-12 ((^{12}_{6}C)) has 6 neutrons.
- Carbon-13 ((^{13}_{6}C)) has 7 neutrons.
- Carbon-14 ((^{14}_{6}C)) has 8 neutrons.
Each of these isotopes of carbon has the same chemical properties due to the same number of protons (atomic number) but differs in mass due to the varying number of neutrons.
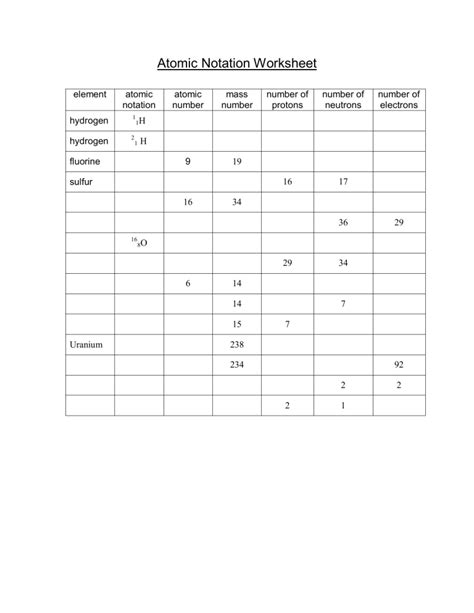
3. Practicing Notation with Examples

Practicing isotope notation with different elements and their isotopes helps reinforce understanding. Let’s try a few examples:
- Oxygen-16: [^{16}_{8}O]
- Nitrogen-14: [^{14}_{7}N]
- Uranium-238: [^{238}_{92}U]
When writing isotope notation, ensure you correctly identify the atomic number (Z) and the mass number (A) for each element.
4. Learning About Applications of Isotopes
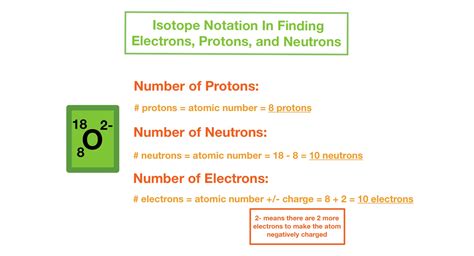
Isotopes have various applications in science, medicine, and industry. Understanding these applications can help deepen your appreciation of isotope notation. For instance:
- Carbon-14 Dating: Used in archaeology to determine the age of ancient organic materials.
- Medical Imaging: Isotopes like oxygen-15, nitrogen-13, and carbon-11 are used in positron emission tomography (PET) scans.
- Industrial Uses: Isotopes are used in the production of food, in semiconductor manufacturing, and in environmental science for tracing water and pollutants.
Knowing the practical applications of isotopes can make learning their notation more engaging and relevant.
5. Utilizing Online Tools and Resources
There are numerous online tools and resources available to help you master isotope notation, including:
- Periodic Tables with Isotope Information: Many online periodic tables provide detailed isotope information for each element.
- Isotope Calculators: Tools that help calculate the mass number of an isotope given the number of protons and neutrons.
- Educational Websites and Videos: Resources that offer step-by-step explanations and exercises to practice writing isotope notation.
Utilizing these resources can provide interactive learning experiences and immediate feedback on your understanding of isotope notation.
Summing up, mastering isotope notation involves understanding its format, recognizing isotopic variations, practicing notation, learning about applications, and utilizing available resources. By following these steps, you’ll be well-equipped to accurately describe and work with isotopes in various scientific contexts.
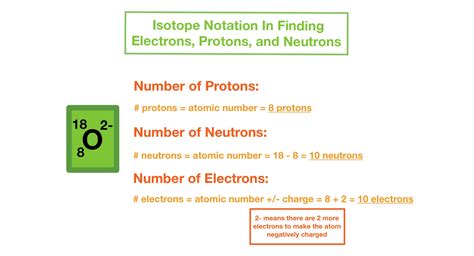
What does the atomic number in isotope notation represent?
+
The atomic number (Z) in isotope notation represents the number of protons in the nucleus of an atom, defining the element.
Why is it important to master isotope notation?

+
Mastery of isotope notation is crucial for accurate scientific communication, identification of isotopes, and understanding their applications in various fields.
Where can I find resources to practice writing isotope notation?

+
Resources for practicing isotope notation include online periodic tables, educational websites, videos, and isotope calculators.
Related Terms:
- Isotope notation worksheet pdf
- Isotope notation worksheet answer key
- Isotope notation worksheet answers pdf
- isotope notation chem worksheet 4 2
- Isotope notation examples
- Atomic notation Worksheet



