5 Essential Tips for Isotope Calculations Worksheet Mastery

If you’re diving into the world of chemistry, particularly into nuclear chemistry or radioactivity, you'll likely encounter isotope calculations. Understanding how isotopes work is fundamental not only for passing exams but also for practical applications in various scientific fields. Let's explore five essential tips that will help you master isotope calculations through worksheets, improving your skills and ensuring your calculations are accurate and efficient.
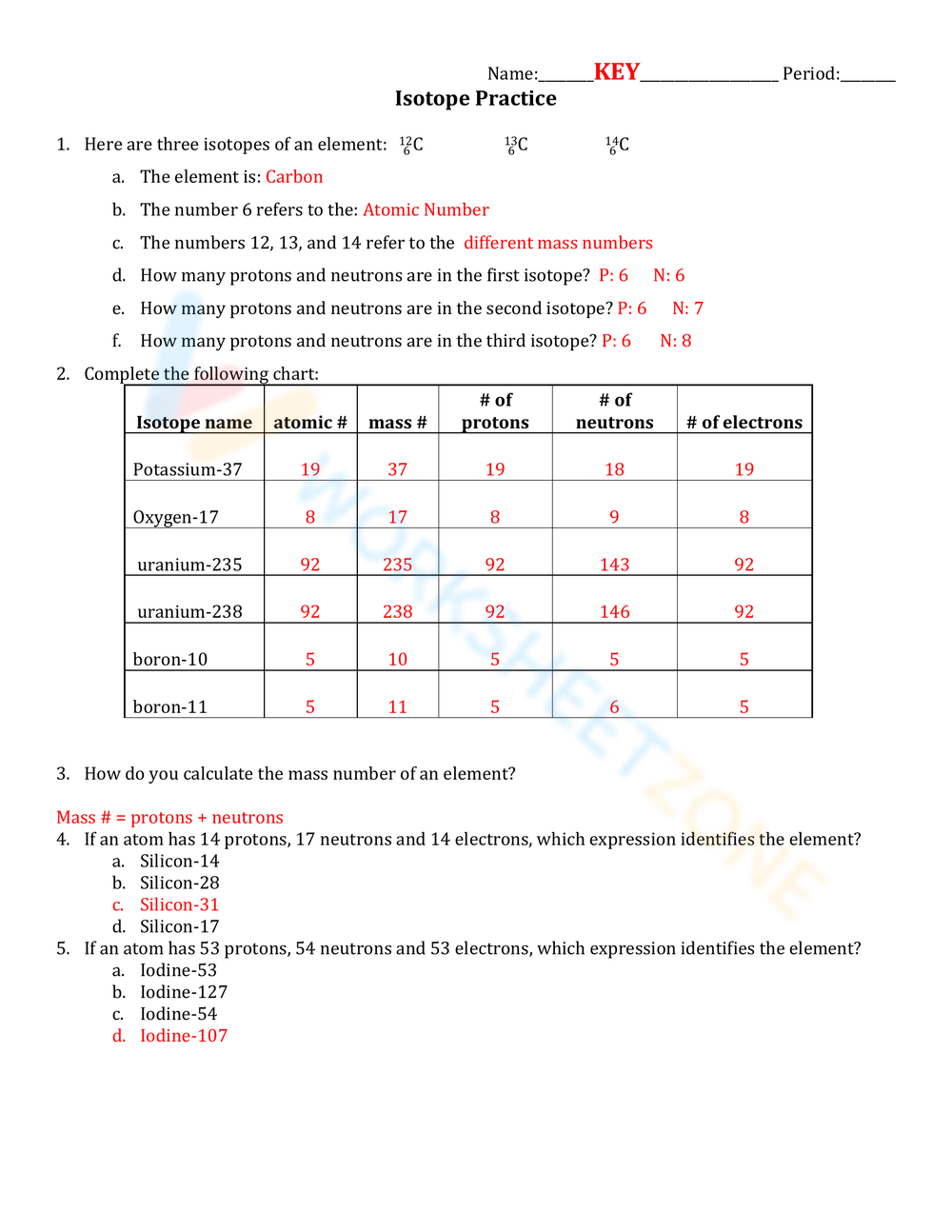
Understand the Basics of Isotopes

Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This variation in neutron count results in different atomic masses for isotopes of the same element. Here’s a quick breakdown:
- Proton - determines the element’s identity.
- Neutron - affects the atomic mass but not the chemical behavior.
- Mass number - total number of protons and neutrons in an atom.
Make sure you can differentiate isotopes from ions (charged particles).
Identify Isotopes Correctly
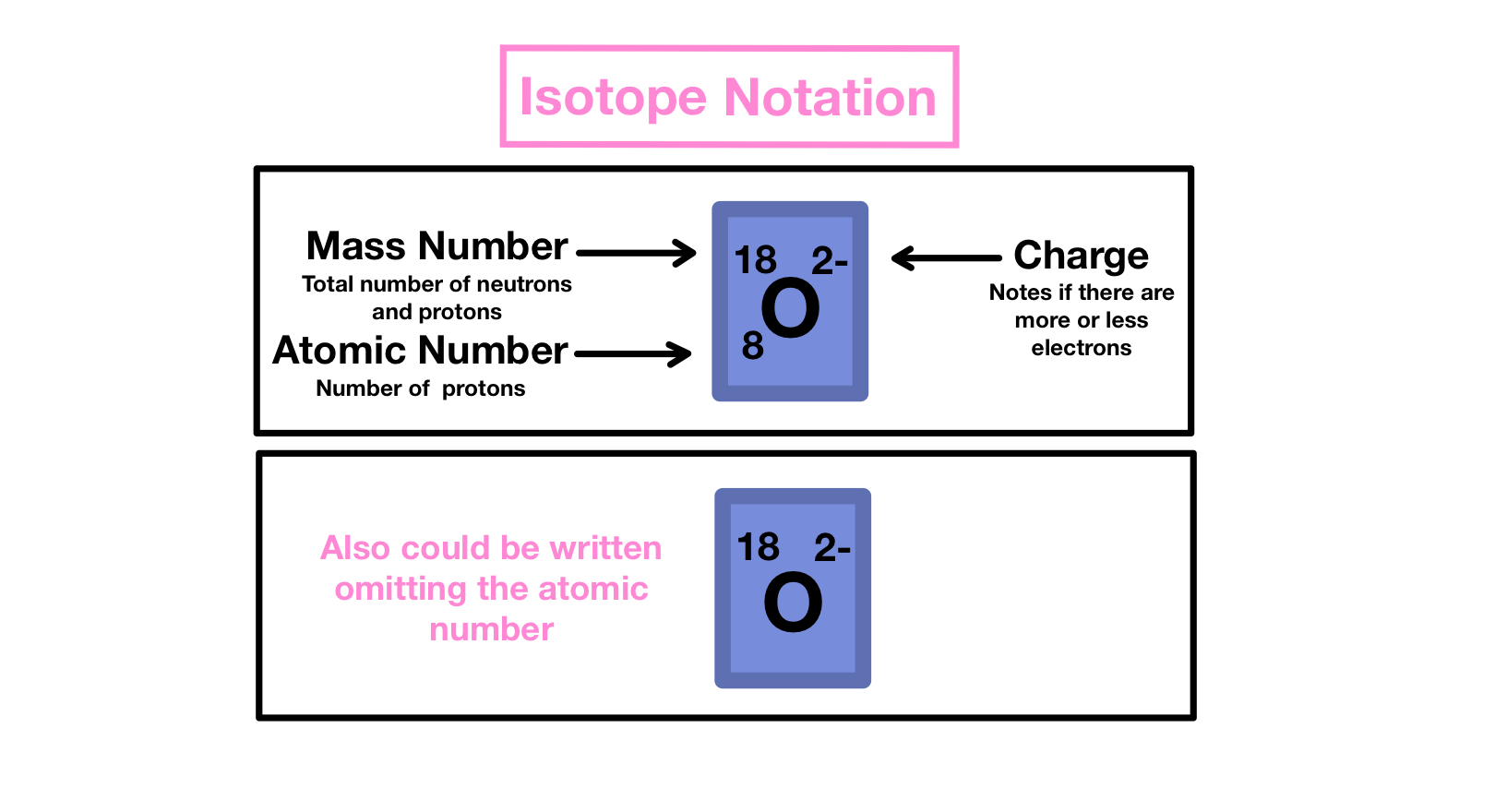
When you work with isotopes in a worksheet, always check:
- The element’s name or symbol.
- The mass number (top left of the isotopic symbol).
- The atomic number (bottom left of the symbol).
- The number of neutrons (mass number - atomic number).
🔬 Note: Some worksheets might only give you partial information, so you must calculate the missing pieces.
Use Atomic Mass Units and Percentage Abundance

Isotopic calculations often involve:
- Atomic Mass: Calculated as the weighted average of the isotopes of an element, where the contribution of each isotope is proportionate to its abundance.
- Isotopic Abundance: The percentage of an isotope present in a naturally occurring sample of an element.
Here’s a simple formula to remember:
Atomic Mass = (Mass of isotope 1 × Abundance) + (Mass of isotope 2 × Abundance) + …
🌡️ Note: Remember to convert percentages to decimals in these calculations.
Practice with a Systematic Approach

When tackling isotope calculations:
- Identify the isotopes involved.
- Note their masses and relative abundance.
- Use the formula above to compute the average atomic mass.
- Double-check your calculations for accuracy.
Here’s an example with Oxygen:
| Isotope | Mass Number | Percentage Abundance |
|---|---|---|
| Oxygen-16 | 15.9949 | 99.757% |
| Oxygen-17 | 17.0 | 0.038% |
| Oxygen-18 | 18.0 | 0.205% |

Using this data:
Atomic Mass of Oxygen ≈ (15.9949 × 0.99757) + (17.0 × 0.00038) + (18.0 × 0.00205) Atomic Mass ≈ 16.009 u
Review and Re-evaluate
After completing each set of calculations:
- Check your work: Ensure your answers make sense given the information provided.
- Compare with published values: Look up the element’s atomic mass in the periodic table to compare with your results.
- Learn from errors: If you made a mistake, analyze where you went wrong and how to avoid similar mistakes.
📝 Note: Regular reviews and practice will enhance your speed and accuracy.
In mastering isotope calculations, it’s not just about crunching numbers but understanding the science behind the calculations. Isotopes have profound implications in fields like medical diagnostics, archaeology, and nuclear power. By honing your skills in isotopic calculations through these worksheets, you equip yourself with knowledge applicable in both theoretical and practical contexts, enhancing your grasp on how elements behave at the atomic level.
What is the difference between an isotope and an element?

+
An element is identified by its number of protons, which defines its chemical properties. An isotope is a variant of an element with a different number of neutrons, leading to differences in mass but not in chemical behavior.
Why is it important to know isotopic abundance for atomic mass calculations?

+
Isotopic abundance helps in calculating the weighted average mass of an element since not all isotopes occur in equal amounts. This weighted average provides the most accurate representation of an element’s mass in nature.
Can the isotopic composition of an element change?

+
Yes, the isotopic composition can vary slightly depending on the source or location. However, for most practical purposes, the natural isotopic abundance is considered constant.
How does understanding isotopes help in real-world applications?

+
Isotopes are used in various fields like medicine for imaging techniques (e.g., PET scans), in geological dating, in nuclear reactors, and even in water and air quality monitoring. Understanding isotopes helps in these applications by allowing for accurate measurement and manipulation of nuclear properties.
Are there any common mistakes to avoid when performing isotopic calculations?

+
Common mistakes include not converting percentages to decimals, overlooking the contribution of less abundant isotopes, or misinterpreting isotopic symbols. Always double-check your calculations and ensure you have all the necessary data.