5 Ways to Master Isotope and Ion Worksheets

In mastering the intricacies of chemistry, understanding isotopes and ions is pivotal. These concepts form the building blocks for grasping more complex chemical behaviors and reactions. This blog post will guide you through five effective strategies to excel in handling isotopes and ion worksheets and enhance your understanding of atomic structure, mass spectrometry, and much more.
Strategy 1: Understanding the Basics of Isotopes and Ions
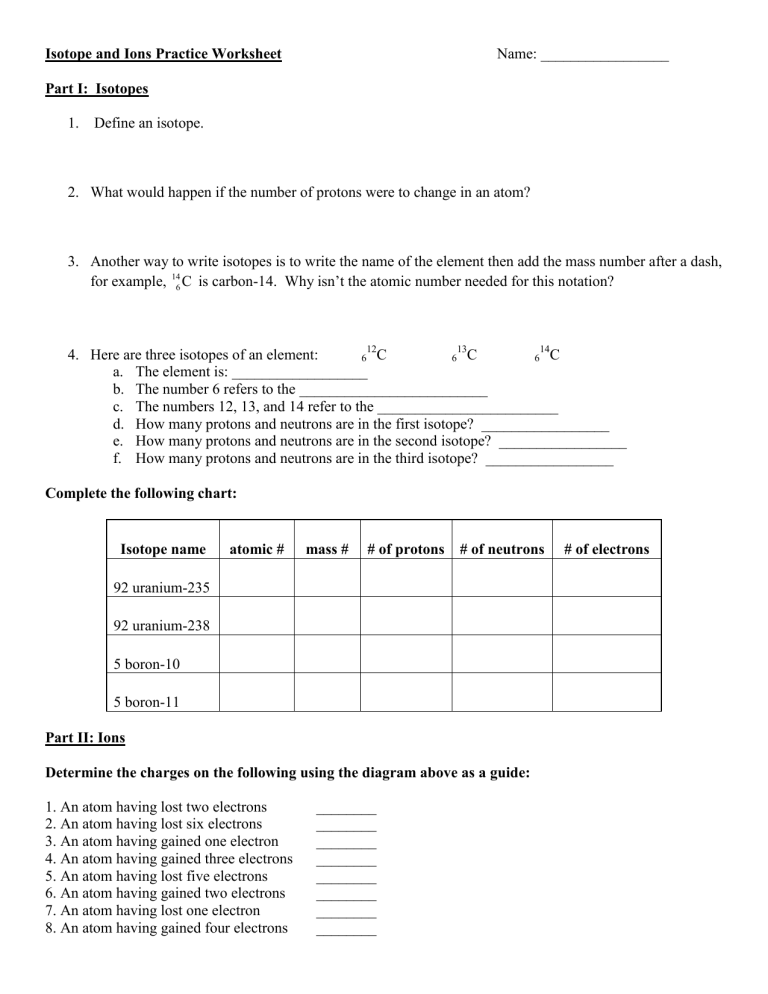

Isotopes are atoms of the same element with different numbers of neutrons. This results in varying atomic masses for the same element. Here's how to master this concept:
- Define Isotopes: Know that isotopes differ in neutron count but have the same atomic number.
- Visualize Atomic Structure: Use diagrams to illustrate how isotopes differ in terms of atomic mass while maintaining the same proton count.
- Practice Notation: Familiarize yourself with isotopic notation, where, for example, Carbon-12 is written as 12C or C-12.
Strategy 2: Identify and Calculate Masses
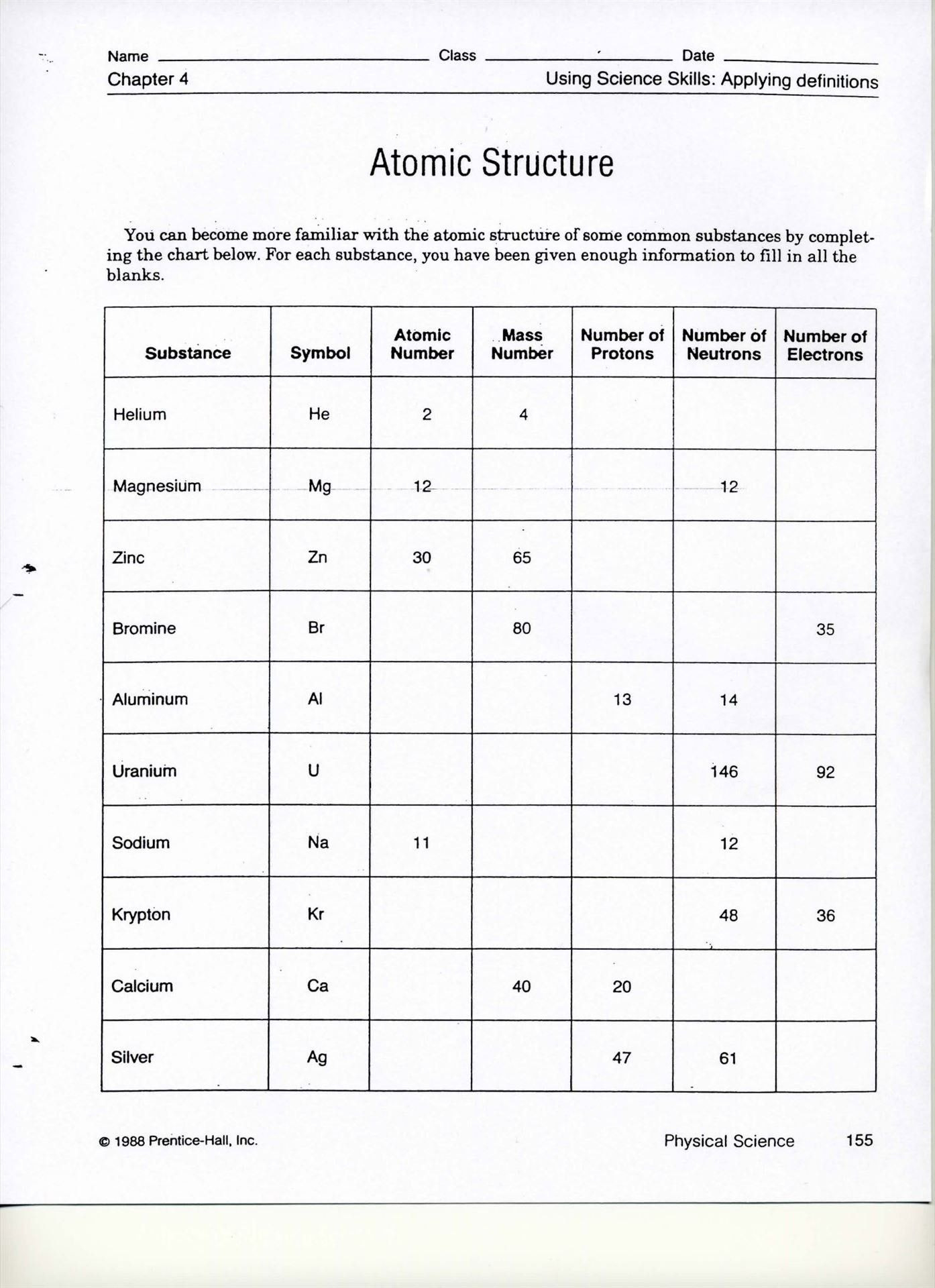
Mass spectrometry is crucial for separating and identifying isotopes. Here's how you can master mass calculations:
- Learn Mass Defect: Understand that the atomic mass listed on the periodic table includes the weighted average of all isotopes of that element.
- Use Mass Spectrometry Data: Interpret graphs or charts to determine the presence and abundance of isotopes.
- Practice Calculations: Engage in exercises where you calculate the average atomic mass from isotope abundances.
Strategy 3: Navigating through Ion Worksheets

Ions, being charged particles, are fundamental in understanding chemical reactions. Here's how to work through ion worksheets effectively:
- Learn Ion Formation: Recognize how atoms gain or lose electrons to form ions, resulting in a net charge.
- Monatomic vs. Polyatomic Ions: Differentiate between these two types of ions. Monatomic ions are individual atoms with a charge, while polyatomic ions are groups of atoms with an overall charge.
- Charge and Naming: Master the rules for naming ions and understanding the charges they typically carry.
Strategy 4: Applying Real-Life Examples

Connecting theoretical knowledge with real-world applications can enhance retention and comprehension:
- Use Case Scenarios: Study real-life examples where isotopes or ions play significant roles, like carbon dating, radiation therapy, or environmental chemistry.
- Scientific Literature: Review articles or textbooks where ions and isotopes are discussed to see how they're applied in various scientific fields.
- Interactive Simulations: Utilize digital tools to simulate the behavior of isotopes or ions in different environments.
Strategy 5: Utilize Collaborative Learning
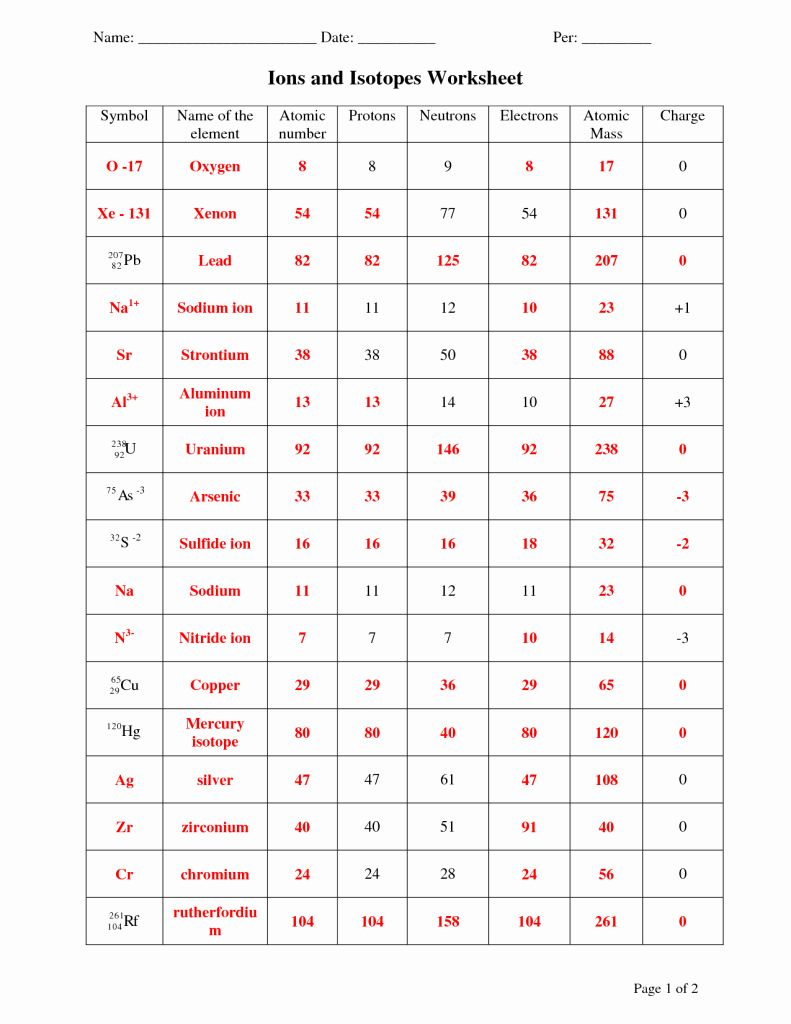
Collaborating with peers can provide different perspectives and insights:
- Group Study Sessions: Join or organize study groups where you can discuss and quiz each other on isotopes and ions.
- Explain to Others: Teaching others can solidify your own understanding; explain the concepts to friends or younger students.
- Peer Review: Exchange worksheets to check each other's work for accuracy and understanding.
By integrating these strategies into your study routine, you'll be better prepared to tackle worksheets on isotopes and ions with confidence. The key is not just to memorize, but to truly understand and apply these concepts in varied contexts.
Remember, mastering these concepts requires consistent practice and a willingness to explore beyond the basic definitions. Here are some important notes to keep in mind:
💡 Note: Isotopes and ions may seem similar due to their atomic nature, but they address different aspects of atomic behavior. Isotopes are about mass variations, while ions are about electrical charge.
⚠️ Note: Always double-check your answers on worksheets. Small mistakes can lead to significant errors in calculations or interpretations.
As you continue your journey in chemistry, remember that isotopes and ions are not just abstract concepts but are integral to understanding the world around us. From medical applications like nuclear medicine to environmental assessments, these building blocks of matter play a crucial role. With practice, application, and collaboration, you'll find yourself navigating through isotopes and ions with ease and confidence, unlocking deeper insights into the fascinating world of chemistry.
What are the key differences between isotopes and ions?

+
Isotopes are variants of an element with different numbers of neutrons but the same number of protons, leading to a change in atomic mass. Ions, on the other hand, are atoms or molecules with an electric charge, gained or lost through the addition or subtraction of electrons.
How can mass spectrometry help in identifying isotopes?

+
Mass spectrometry measures the mass-to-charge ratio of ions. By analyzing the mass distribution, scientists can determine the presence and relative abundance of different isotopes of an element.
Why is it important to understand isotopes in real-life scenarios?
+
Understanding isotopes is crucial for applications like carbon dating, where isotope ratios are used to date archaeological or biological specimens, in nuclear medicine for tracing the path of substances, and in environmental science for tracking pollution sources or understanding climate change.