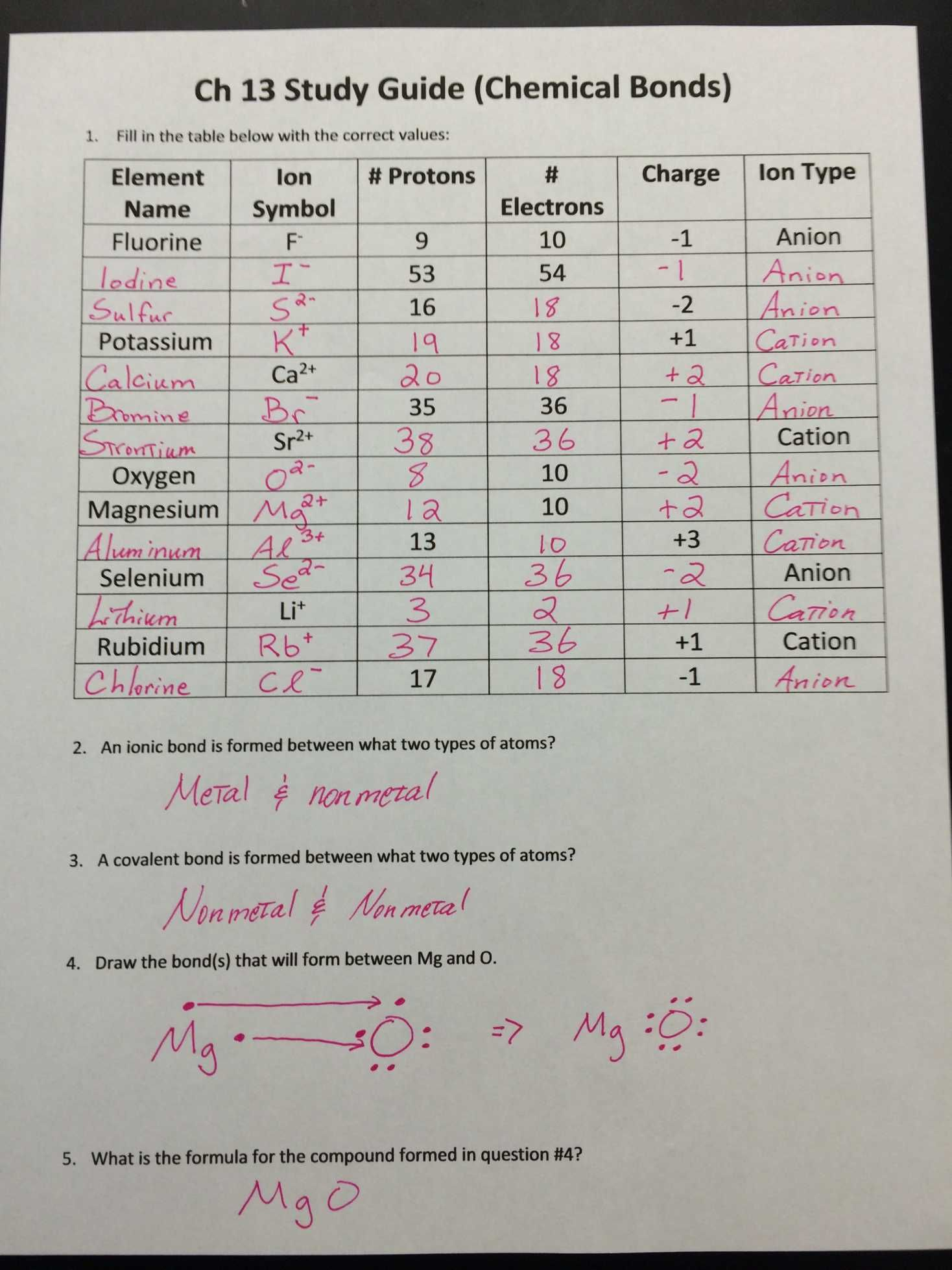
Ions Worksheet Answer Key: Unlock Chemistry Mastery Now

Unlocking chemistry mastery can be as exhilarating as it is essential, particularly when dealing with the intricate world of ions. Understanding ions is a key component in unlocking the broader mysteries of chemistry, from how atoms bond to form compounds to how reactions occur in both natural and lab settings. Today, we delve into an "Ions Worksheet Answer Key" which will not only clarify the concepts of ions but also provide you with the tools necessary to excel in this fundamental area of chemistry.
The Basics of Ions

Before we dive into the specifics of the worksheet, let's define what ions are. An ion is an atom or molecule that has either gained or lost one or more electrons, thereby acquiring a net electric charge. Here's how we classify them:
- Anions: These are negatively charged ions formed when atoms gain one or more electrons.
- Cations: Positively charged ions occur when atoms lose one or more electrons.
These charged particles play pivotal roles in chemical reactions, electrolyte solutions, and are crucial in understanding the behavior of elements in various environments.
How Ions are Formed

Ion formation can be explained through several fundamental concepts:
- Valence electrons dictate the likelihood of an atom to form ions. Elements with 1, 2, or 3 electrons in their valence shell tend to lose these to achieve a stable electron configuration, becoming cations.
- Similarly, elements with 6, 7, or 8 valence electrons are likely to gain electrons to fill their outermost shell, becoming anions.
Here are some common examples:
| Element | Atomic Number | Typical Ion | Charge |
|---|---|---|---|
| Sodium (Na) | 11 | Na+ | +1 |
| Fluorine (F) | 9 | F- | -1 |
| Calcium (Ca) | 20 | Ca2+ | +2 |
| Oxygen (O) | 8 | O2- | -2 |

Identifying and Naming Ions

Understanding how to name and identify ions is crucial:
- Cations: The name remains the same as the element's name but ends in "-ion". For instance, sodium becomes sodium ion.
- Anions: The stem of the element's name changes, typically ending in "-ide". For example, fluorine becomes fluoride ion.
- Polyatomic ions: These are ions composed of more than one atom, and their naming can vary, but they often have specific names (e.g., NH4+ is ammonium).
💡 Note: Always ensure to consider the overall charge of compounds involving polyatomic ions when balancing chemical equations.
Working Through the Worksheet

The "Ions Worksheet Answer Key" we're focusing on will help solidify your understanding of ions through practical exercises. Here's a breakdown of typical questions you might find:
Question 1: Ionic Charges

- Given an element's name or symbol, determine its most likely ionic charge.
Example:
- Potassium (K): +1
- Sulfur (S): -2
Question 2: Anion and Cation Identification

- Identify the anion and cation in a given compound.
Example:
- Magnesium Chloride (MgCl2): Magnesium is the cation (Mg2+), and Chloride is the anion (Cl-).
Question 3: Balancing Ionic Charges

- Determine how many ions are necessary to form neutral compounds.
Example:
- Aluminum Sulfide (Al2S3): Aluminum has a +3 charge, and Sulfide has a -2 charge. Two aluminum ions are needed to balance three sulfur ions.
Question 4: Nomenclature

- Provide the chemical formula and name for various ions.
Example:
- Fe2+: Iron(II) or ferrous.
- CrO42-: Chromate ion.
Question 5: Compounds and Predicting Products

- Given reactants, predict the products of ionic reactions.
Example:
- NaOH (aq) + FeCl3 (aq) →: Fe(OH)3 (s) + NaCl (aq)
🚨 Note: Remember that solubility rules play a significant role in predicting the products of ionic reactions.
Advanced Topics

Beyond the basics, there are several advanced concepts related to ions:
- Electrolytes: Compounds that dissociate in solution to produce ions, which conduct electricity.
- Ion Exchange: The process of exchanging ions between two phases, commonly used in water softening and other purification processes.
- Redox Reactions: Reactions where oxidation and reduction occur simultaneously, often involving electron transfer.
Understanding these can further your knowledge and application of ion chemistry:
Electrolytes and Ion Conductivity
- Some key points:
- Strong electrolytes dissociate completely into ions in solution, while weak electrolytes only partially dissociate.
- Electrolyte concentration affects the conductivity of solutions.
Ion Exchange Applications

- Applications include:
- Water softening through removal of calcium and magnesium ions using sodium-rich ion exchange resins.
- Purification processes in labs and industries.
Redox Reactions

- Key aspects:
- Redox involves the transfer of electrons between reactants, leading to a change in oxidation states.
- Balancing redox reactions requires knowledge of both ionic charges and mass conservation.
📝 Note: Redox reactions are fundamental to understanding electrochemistry and battery technology.
Summing Up

In mastering ions, we unlock the key to a deeper understanding of chemical interactions, from how compounds form to how they react in various conditions. Through our journey with the "Ions Worksheet Answer Key", you've not only learned the basics of ion formation and identification but also explored advanced applications and implications of ionic chemistry. This comprehensive knowledge provides a solid foundation for further studies in chemistry or related fields, making you more adept at tackling complex chemical problems. Whether for academic pursuits or practical applications in industries like pharmaceuticals or environmental science, your grasp of ions will prove invaluable.
What’s the difference between an atom and an ion?
+An atom is neutral with an equal number of protons and electrons. An ion has a charge because it has either gained or lost electrons.
Why do some elements tend to lose electrons while others gain them?
+Elements want to achieve a stable electron configuration. Those with 1-3 valence electrons tend to lose them to become stable, while those with 6-8 electrons gain them for stability.
How do I know if an ion is a cation or anion?
+Cations are positively charged, which means they’ve lost electrons. Anions are negatively charged, having gained electrons.
What are polyatomic ions?
+Polyatomic ions are ions composed of two or more atoms covalently bonded together, carrying a net charge.
Can you explain ion exchange?
+Ion exchange is a process where ions are exchanged between two phases (usually a solid and a liquid). It’s used in applications like water softening, where undesirable ions (like Ca2+) are replaced by more desirable ones (like Na+).