Ionic Compounds Worksheet Answers: Ace Your Chemistry Homework
Understanding ionic compounds is fundamental for mastering chemistry, whether you're a high school student or delving into advanced chemical sciences. This detailed guide provides answers to common questions and problems found in Ionic Compounds Worksheets, helping you ace your chemistry homework and grasp the underlying principles of ionic bonds, structures, and reactions.
What Are Ionic Compounds?

Ionic compounds are a type of chemical compound where atoms from different elements are bonded together through ionic bonding. This occurs when one atom loses electrons, forming a positive ion (cation), and another gains electrons, becoming a negative ion (anion). Here are some key characteristics:
- Electrostatic Attraction: Ionic bonds result from the attraction between oppositely charged ions.
- High Melting Points: Due to the strong ionic forces, these compounds typically have high melting points.
- Soluble in Water: Many ionic compounds dissolve in water, where ions can move freely.

Forming Ionic Bonds

Here’s how ionic bonds form:
- Atom Ionization: One atom loses electrons to become a cation, another gains electrons to become an anion.
- Electron Transfer: Electrons are transferred from the metal to the non-metal.
- Electrostatic Attraction: The resulting oppositely charged ions attract each other, forming the ionic bond.
💡 Note: The octet rule is often satisfied in this process, aiming for the stability of a noble gas configuration.
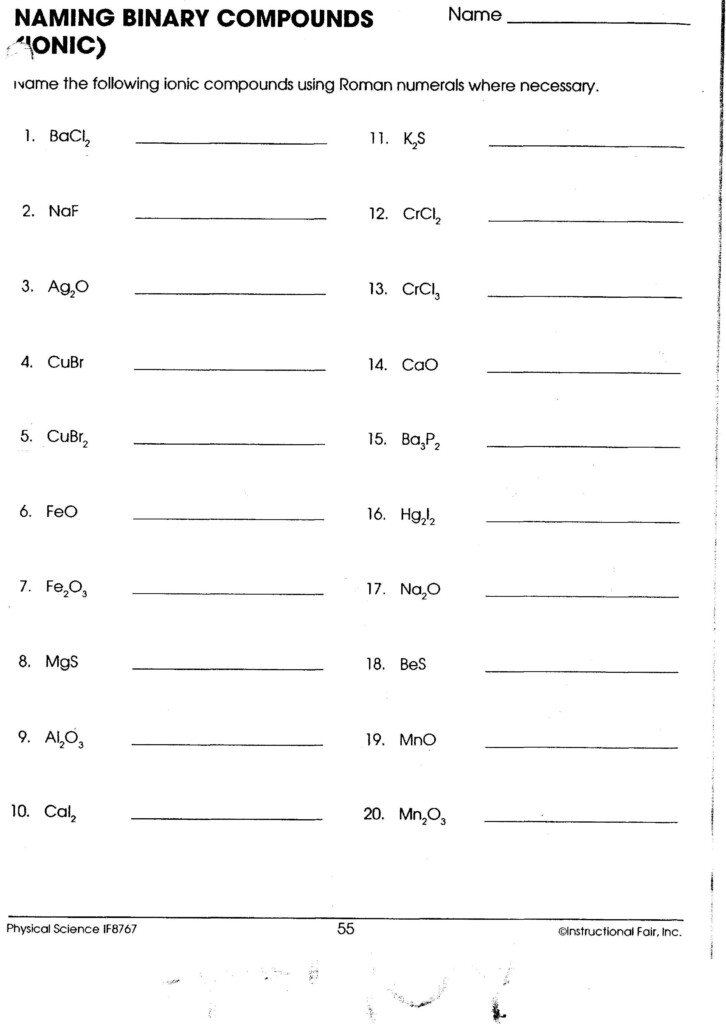
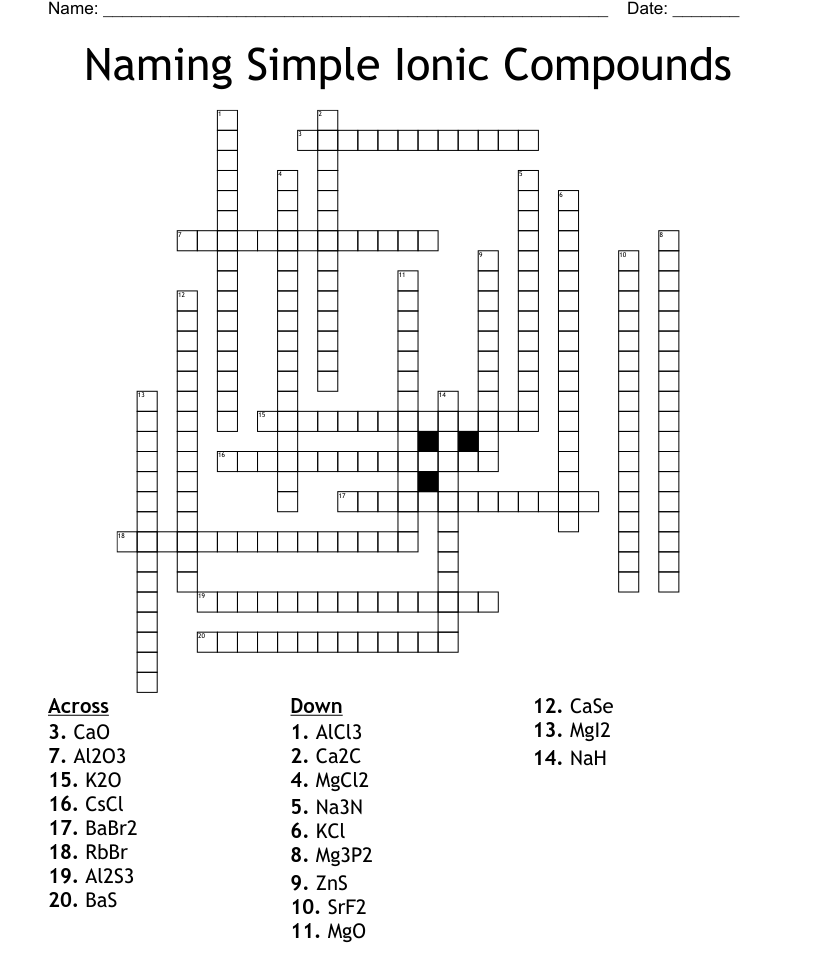
Naming Ionic Compounds

The rules for naming ionic compounds are fairly straightforward:
- Cation First: Always name the positively charged ion (metal or polyatomic ion) first.
- Anion: Follow with the name of the anion, adding the suffix “-ide” to the element’s root name.
- Transition Metals: Include Roman numerals in parentheses after the metal’s name to indicate its charge if there is more than one possible charge.
Here’s an example:
- NaCl: Sodium chloride
- Fe₂O₃: Iron(III) oxide
Writing Ionic Formulas

To write the chemical formula of an ionic compound, follow these steps:
- Determine Charges: Find out the charge on each ion based on their periodic table location or known charge.
- Balance Charges: Use the criss-cross method to balance the charges. The cation’s charge becomes the anion’s subscript, and vice versa.
- Simplify: Reduce the subscripts if possible to make the smallest whole number ratio.
| Compound | Ions | Formula |
|---|---|---|
| Calcium chloride | Ca²⁺, Cl⁻ | CaCl₂ |
| Aluminum oxide | Al³⁺, O²⁻ | Al₂O₃ |

📚 Note: When balancing charges, always simplify to find the lowest common ratio.
Properties of Ionic Compounds

Ionic compounds have several distinctive properties:
- High Melting Points: The ionic bonds require a lot of energy to break.
- Crystalline Structure: They form regular, repeating patterns in the solid state.
- Brittleness: When stress is applied, the layers of ions can slide, causing the crystal to shatter.
- Electrical Conductivity: Conduct electricity when molten or dissolved in water due to mobile ions.
Practical Applications

Ionic compounds are not just academic; they have practical uses in daily life:
- Sodium Chloride (NaCl): Used in food preservation, flavoring, and melting ice.
- Magnesium Sulfate (MgSO₄): Known as Epsom salts, used in baths for relaxation.
- Potassium Nitrate (KNO₃): Used in fertilizers, explosives, and as a meat curing agent.
In conclusion, this comprehensive guide to ionic compounds not only provides answers to common worksheet questions but also delves into the underlying principles, naming conventions, and practical applications of ionic compounds. Understanding these fundamental concepts can make chemistry not only easier to grasp but also more interesting, highlighting the intricate beauty of molecular interactions. By mastering these principles, you're well on your way to excelling in your chemistry studies, equipped with the knowledge to tackle more complex topics with confidence.
What are the main differences between ionic and covalent compounds?

+
Ionic compounds involve the transfer of electrons resulting in charged ions that form ionic bonds, while covalent compounds share electrons, forming covalent bonds. Ionic compounds typically have high melting points and are soluble in water, whereas covalent compounds might have lower melting points and solubility varies widely.
How do you know when to use Roman numerals when naming ionic compounds?
+
Roman numerals are used when naming compounds with transition metals that can have multiple charges. They indicate the charge of the cation, such as in Iron(II) oxide (FeO) and Iron(III) oxide (Fe₂O₃).
Why do ionic compounds dissolve in water?

+
Water is a polar molecule, and its partial charges interact with the ions of ionic compounds, pulling them apart from the solid lattice structure. This solvation process allows the ions to move freely in the solution, enabling ionic compounds to dissolve.



