Ionic Practice Worksheet Answers: Boost Your Skills Easily

In the realm of chemistry, understanding ion formation is crucial for mastering the concepts related to chemical reactions, bonding, and properties of elements. This blog post aims to provide an exhaustive look into ionic practice worksheet answers, offering insights and strategies to boost your skills in dealing with ions. Whether you're a student grappling with these concepts or an enthusiast looking to deepen your understanding, this guide will serve as a comprehensive resource.
What are Ions?
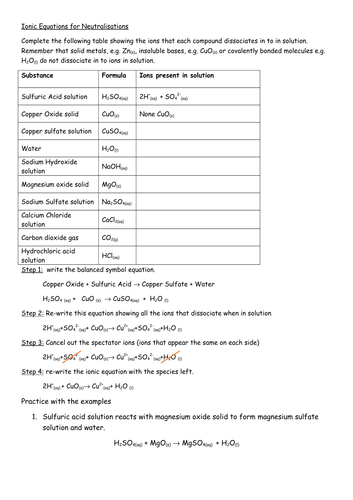
Before diving into the worksheet answers, let’s clarify what ions are. Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge. There are two primary types:
- Cations: Positively charged ions formed when an atom loses one or more electrons. For instance, sodium (Na) loses one electron to form Na+.
- Anions: Negatively charged ions formed when an atom gains one or more electrons. Chlorine (Cl) gains one electron to become Cl-.

How to Identify Ions

To effectively tackle ionic practice worksheets, you need to recognize the patterns in electron configuration and valency:
- Elements from group 1 will always lose one electron to achieve stability, becoming cations.
- Group 17 (halogens) elements often gain one electron, forming anions.
- Transition metals can have multiple oxidation states, making their ion identification trickier.
Practice Worksheet Answers
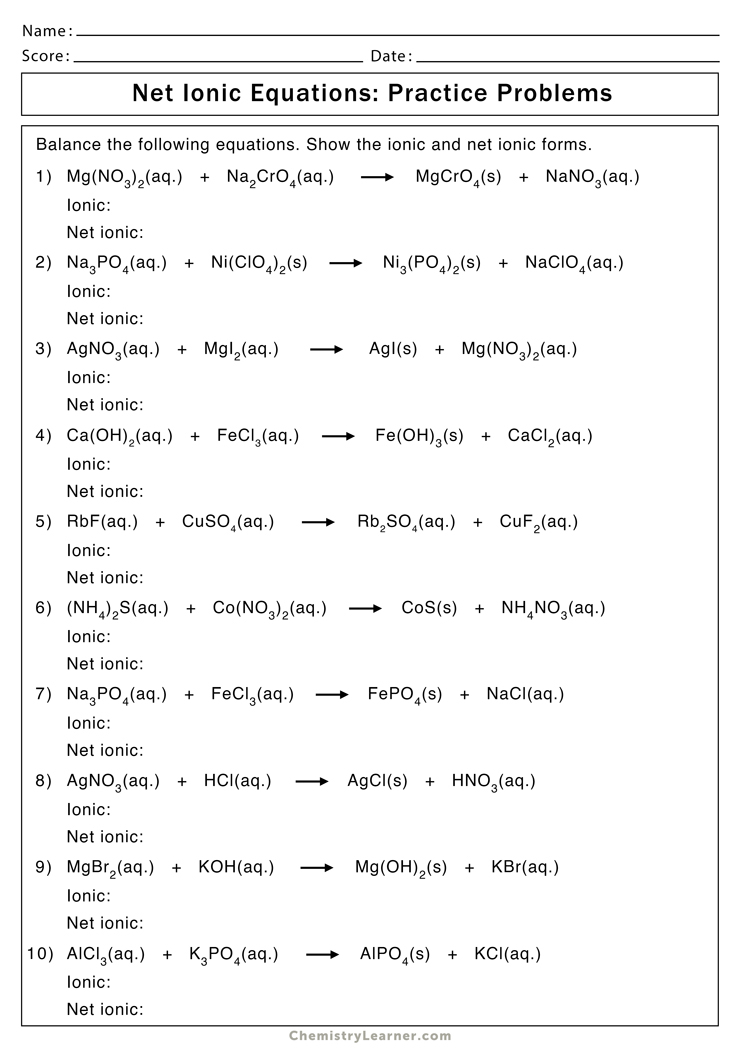
Let’s now delve into the specifics of answering an ionic practice worksheet. Here are some common questions and their answers:
1. Predicting the Ions

When given an element:
- Identify its group number to predict its likely charge.
- Elements like potassium (K) from group 1 form K+, whereas oxygen (O) from group 16 usually forms O2-.
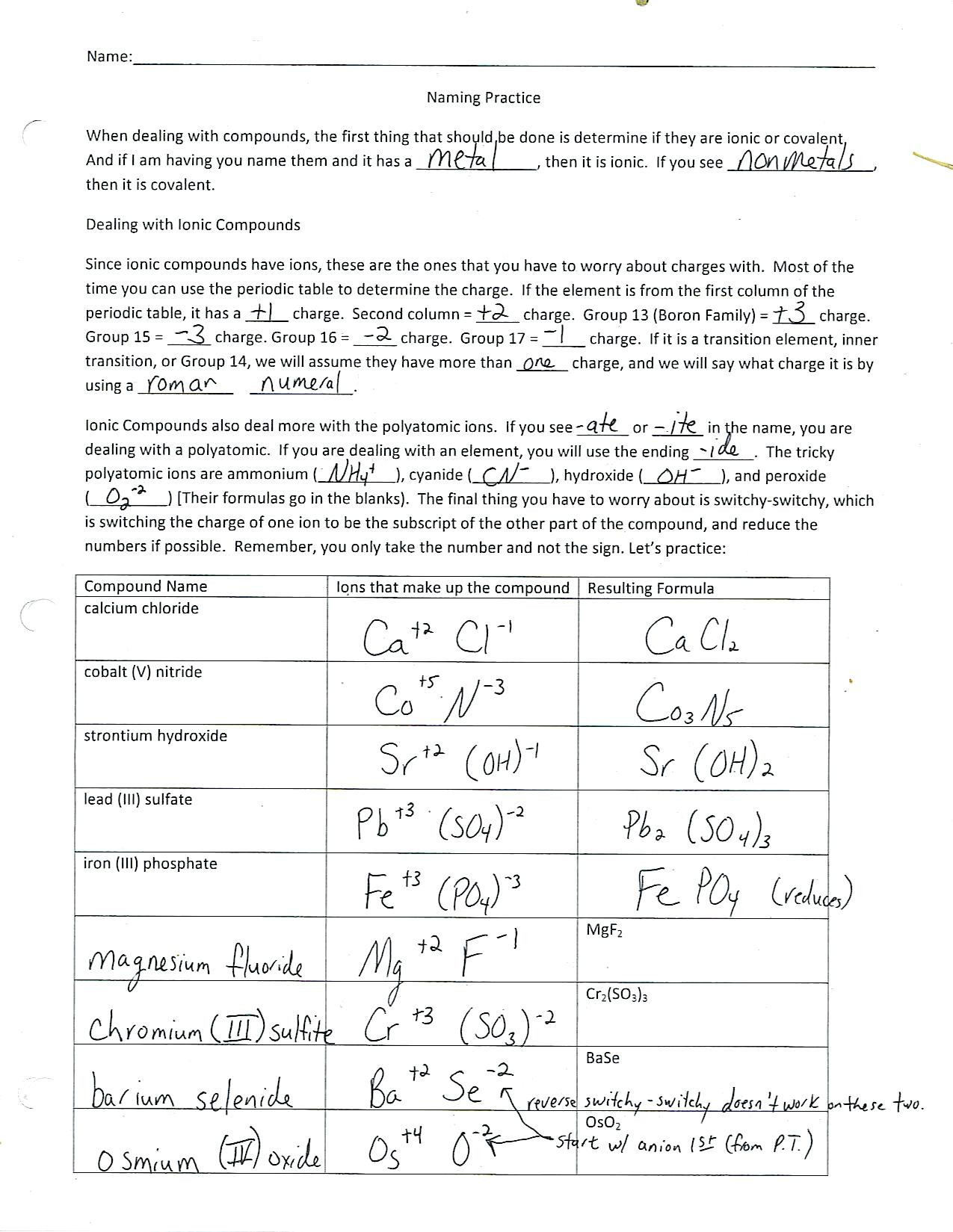
2. Writing Ionic Formulas
Here’s how to write ionic formulas:
- Combine Cations and Anions: Write the cation first followed by the anion.
- Balancing Charges: Balance the charges to form a neutral compound. For example, Al3+ and O2- combine to form Al2O3 to balance the charges (3 from Al and 2 from O, leading to a neutral molecule).
⚠️ Note: Transition metals might have varying charges, so check the given charge or assume the common oxidation state.
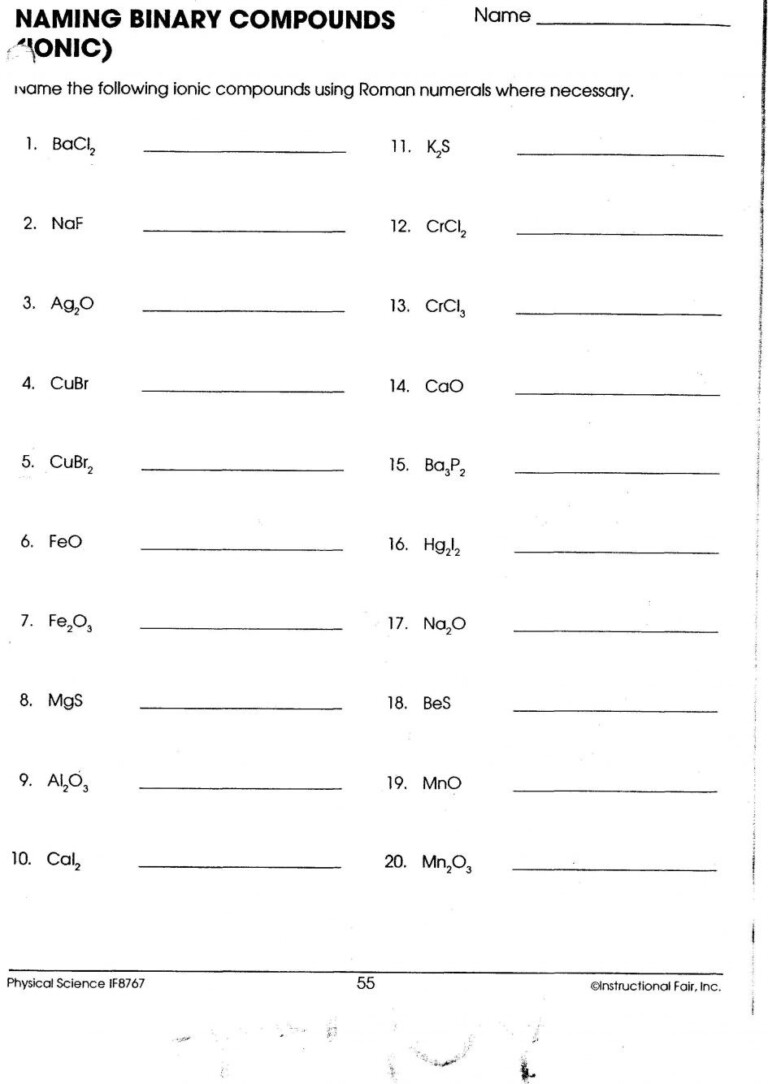
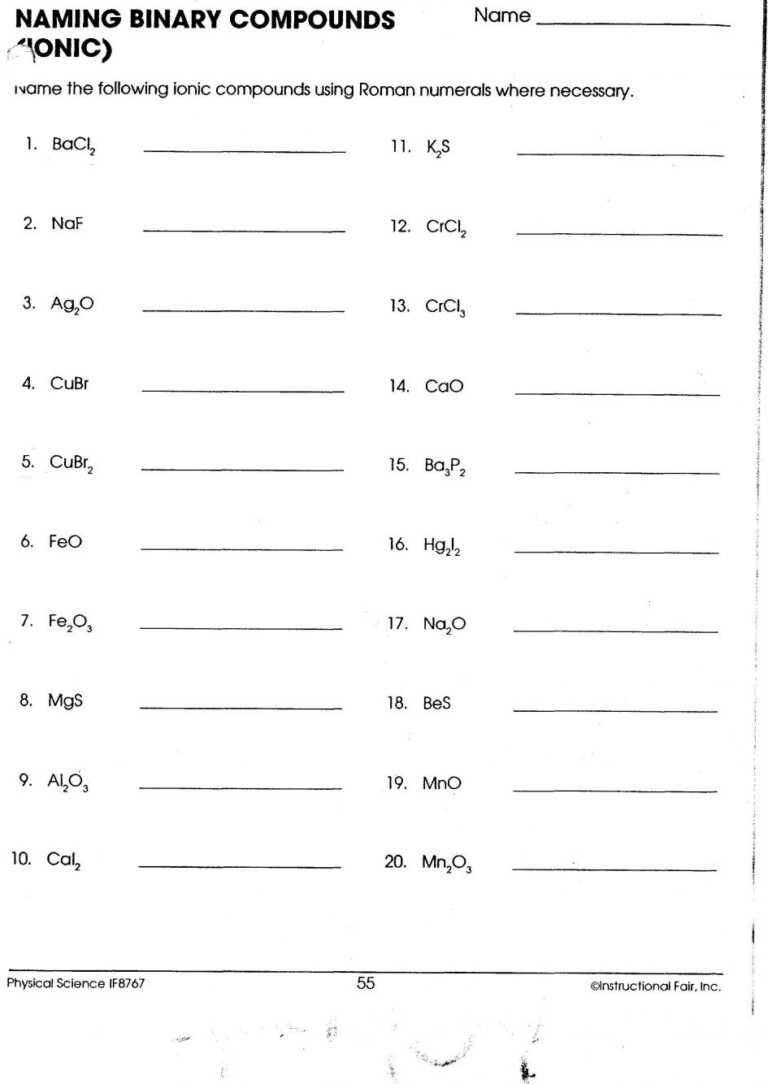
3. Naming Ionic Compounds

Here’s a simple guide:
- Use the name of the cation without changing it.
- For anions, drop the ending and add -ide for single-element ions, or use the polyatomic ion names.
- Example: NaBr (Sodium Bromide) or FeCl3 (Iron(III) Chloride, indicating Fe3+).
| Ionic Compound | Name |
|---|---|
| KCl | Potassium Chloride |
| MgO | Magnesium Oxide |
| Fe(NO3)2 | Iron(II) Nitrate |
Strategies to Boost Your Skills
To enhance your proficiency with ionic compounds:
- Memorize: Common ions and their charges to speed up your process of solving worksheets.
- Practice: Regularly engage with ionic practice worksheets to reinforce your understanding.
- Visualize: Use periodic tables and ion charts to help visualize and remember the relationships between elements and their ions.
- Understand Trends: Familiarize yourself with periodic trends to predict ion formation behaviors.
In wrapping up, the journey through the world of ions and ionic compounds is not just about memorizing facts but understanding the fundamental principles of chemistry. By consistently practicing with ionic worksheets, employing the strategies outlined above, and understanding ion formation, you’ll find that the subject becomes less daunting. As we’ve explored, ions play a pivotal role in countless chemical processes, from simple salt formation to complex biochemical reactions within our bodies. The ability to predict, name, and understand ions will not only boost your academic performance but also enrich your appreciation for the natural world’s intricacies.
Why do some elements form multiple types of ions?

+
Transition metals, in particular, can exhibit multiple oxidation states because their electrons can occupy different energy levels, allowing them to lose or gain electrons in varied ways to achieve stability.
How can I quickly determine the formula of an ionic compound?
+
Look at the charges of the ions involved. Use the “criss-cross” method, where you write down the charges, then use these numbers to create the formula. However, always balance the charges to form a neutral compound.
What are polyatomic ions, and how should I memorize them?
+
Polyatomic ions are molecular ions that contain two or more atoms of different elements. They are often memorized through flashcards, songs, or grouping them by similar endings like ‘ate’ for high oxygen number or ‘ite’ for lower oxygen number.