5 Essential Tips for Mastering Ionic Equations

Balancing ionic equations is a crucial skill for anyone delving into chemistry, particularly in fields like electrochemistry, environmental science, and redox chemistry. Ionic equations aren't just numbers and formulas; they represent the dynamic world of chemical reactions at the ionic level. Understanding and mastering how to balance these equations not only helps in academic learning but is also fundamental for research and industrial applications. In this detailed guide, we'll explore five essential tips that will equip you to tackle ionic equation balancing with confidence.
Tip 1: Identify the Ionic Species


Before diving into balancing, ensure you identify the ionic species involved in the reaction. This means:
- Determining the state of each compound (solid, liquid, gas, or aqueous).
- Separating ionic compounds into their constituent ions.
- Accounting for polyatomic ions which remain intact during the reaction.
Take for example the reaction between silver nitrate (AgNO₃) and potassium chloride (KCl):
- AgNO₃(aq) -> Ag⁺(aq) + NO₃⁻(aq)
- KCl(aq) -> K⁺(aq) + Cl⁻(aq)
By recognizing the ionic species, you set a solid foundation for balancing the equation.
🔬 Note: Not all compounds dissociate into ions when dissolved in water; some remain as molecules.
Tip 2: Balance the Equation Using the Half-Reaction Method


The half-reaction method is the gold standard for balancing redox (reduction-oxidation) reactions:
- Write down the two half-reactions for oxidation and reduction.
- Balance atoms other than hydrogen and oxygen in each half-reaction.
- Balance oxygen by adding water molecules.
- Balance hydrogen by adding H⁺ ions.
- Balance the charges by adding electrons (e⁻) to the side with the higher charge.
- Ensure the number of electrons is the same in both half-reactions, then combine them.
Here’s an example with the reaction of chromium oxide with chlorine:
| Step | Reduction | Oxidation | |
|---|---|---|---|
| 1. Write half-reactions | Cr₂O₇²⁻(aq) + 14H⁺(aq) + 6e⁻ -> 2Cr³⁺(aq) + 7H₂O(l) | Cl₂(g) + 2e⁻ -> 2Cl⁻(aq) | |
| 2. Balance atoms | (already balanced) | (already balanced) | |
| 3. Balance oxygen | (already balanced) | (not needed) | |
| 4. Balance hydrogen | (already balanced) | (not needed) | |
| 5. Balance charge | (6e⁻) | (2e⁻) | |
| 6. Combine half-reactions | Cr₂O₇²⁻(aq) + 14H⁺(aq) + 6Cl⁻(aq) -> 2Cr³⁺(aq) + 3Cl₂(g) + 7H₂O(l) | (final balanced equation) | |

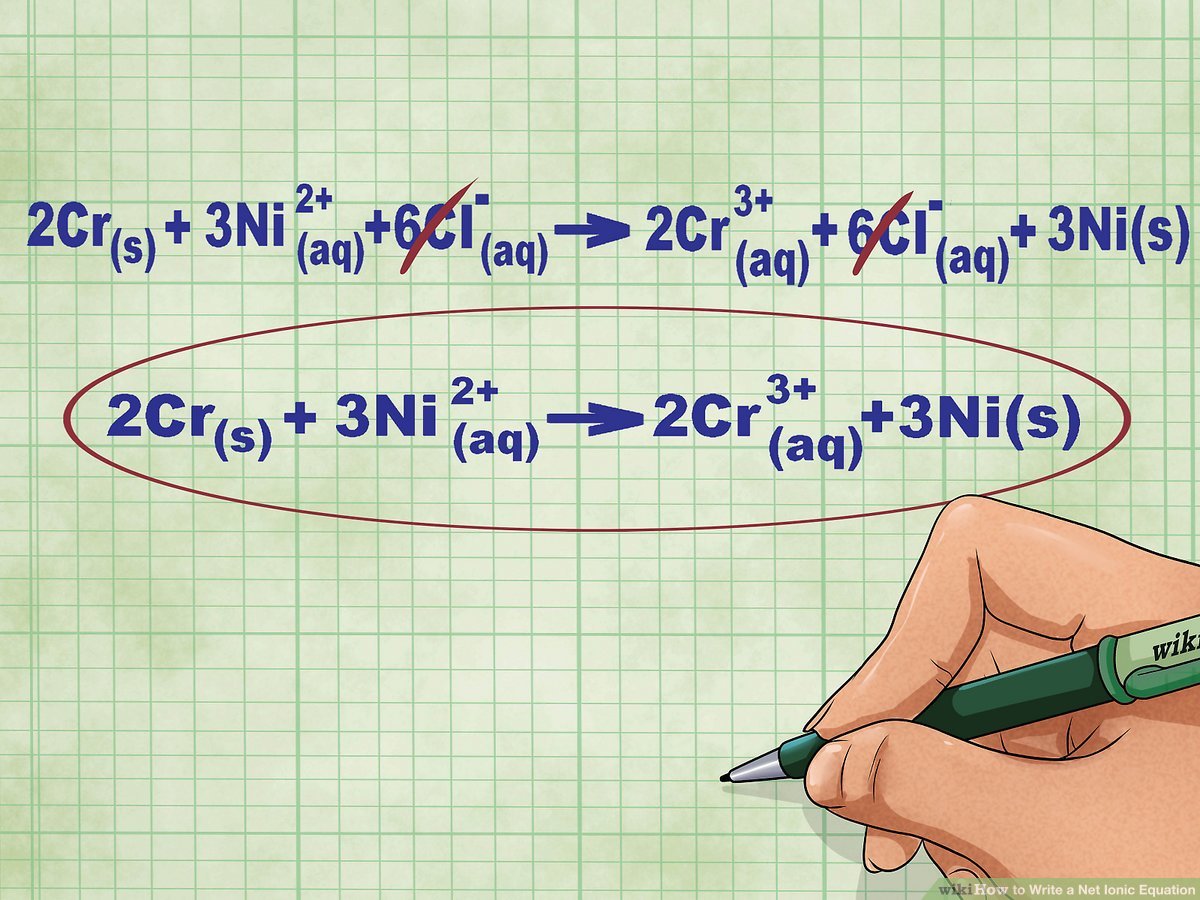
Tip 3: Neutralize Spectator Ions


Spectator ions are ions that don’t participate in the actual chemical reaction. To simplify the equation, they can be canceled out:
- Identify the spectator ions by looking at the ionic equation before and after the reaction.
- These ions remain in the same form on both sides of the equation.
- Eliminate them to get the net ionic equation, which shows only the species involved in the reaction.
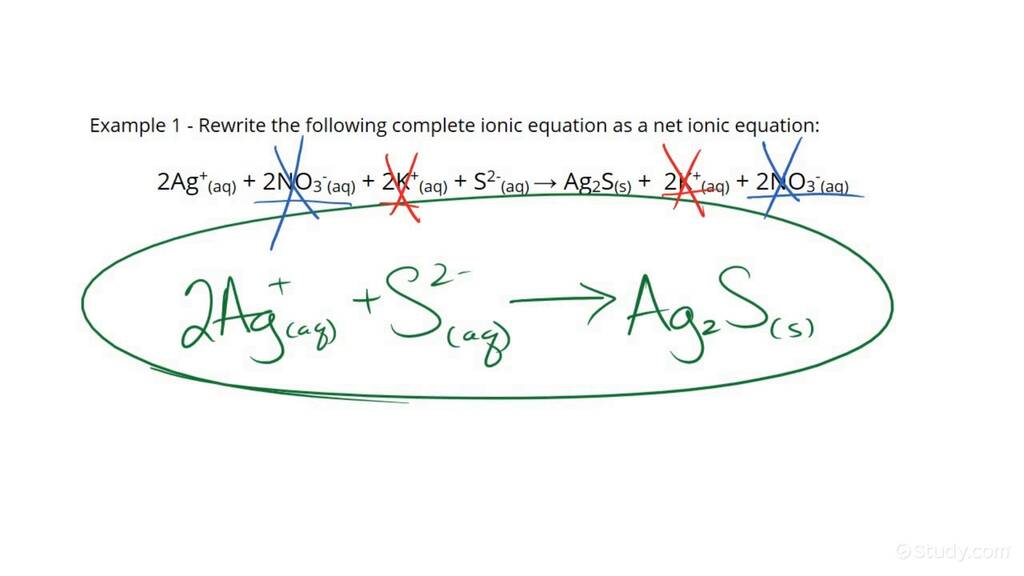
Using the previous example:
Ag⁺(aq) + NO₃⁻(aq) + K⁺(aq) + Cl⁻(aq) -> AgCl(s) + K⁺(aq) + NO₃⁻(aq)
The spectator ions are K⁺ and NO₃⁻, resulting in:
Ag⁺(aq) + Cl⁻(aq) -> AgCl(s)
Tip 4: Practice with Real-World Scenarios

To truly master ionic equations, practice with scenarios you might encounter in real life:
- Water treatment processes
- Corrosion and rust prevention
- Acid-base neutralization in industrial settings
- Battery operation and design
Here’s an exercise for you:
Suppose a water treatment facility wants to neutralize acid mine drainage (FeSO₄ solution) with calcium hydroxide (Ca(OH)₂). How would you balance this?
💡 Note: Applying theoretical knowledge to practical applications helps in understanding the impact and relevance of balancing equations.
Tip 5: Use Online Tools and Apps for Verification


While practicing, use online tools and apps like:
- Chemical Equation Balancer
- Redox Calculator
- ChemBalancer
These tools can verify your balancing work, provide alternative methods, or help with complex reactions that might be challenging to balance manually:
🔎 Note: Tools are aids for learning; understanding the process is still paramount.
By incorporating these five tips into your learning strategy, you’ll find that balancing ionic equations becomes less daunting and more of an engaging puzzle to solve. From identifying the ionic species to utilizing online tools, each tip builds on your understanding and proficiency in ionic chemistry. Remember, mastering ionic equations is a journey of practice, understanding the underlying chemistry, and applying these skills to real-world scenarios.
Let’s address some commonly asked questions:
Why is it necessary to balance ionic equations?
+
Balancing equations ensures conservation of mass and charge in chemical reactions, which are fundamental principles in chemistry. It also allows for accurate prediction and control of reactions.
Can spectator ions ever change in a chemical reaction?

+
In typical redox reactions, spectator ions remain unchanged, but under specific conditions, like extreme pH changes or complexation, they might participate.
How can I improve my speed in balancing complex equations?

+
Practice regularly, learn different balancing methods (like the algebraic method), and use tools to check your work for faster learning.
Is it okay to use online tools for homework?

+
Online tools can be used as learning aids, but understanding the balancing process is crucial. Use tools for verification, not as a crutch.
What should I do if an ionic equation cannot be balanced?

+
Check your work for common mistakes, ensure all ions are correctly identified, or consult resources for complex reactions like dismutation reactions, which might need special approaches.