Master Ionic Equations with Our Easy Worksheet Guide
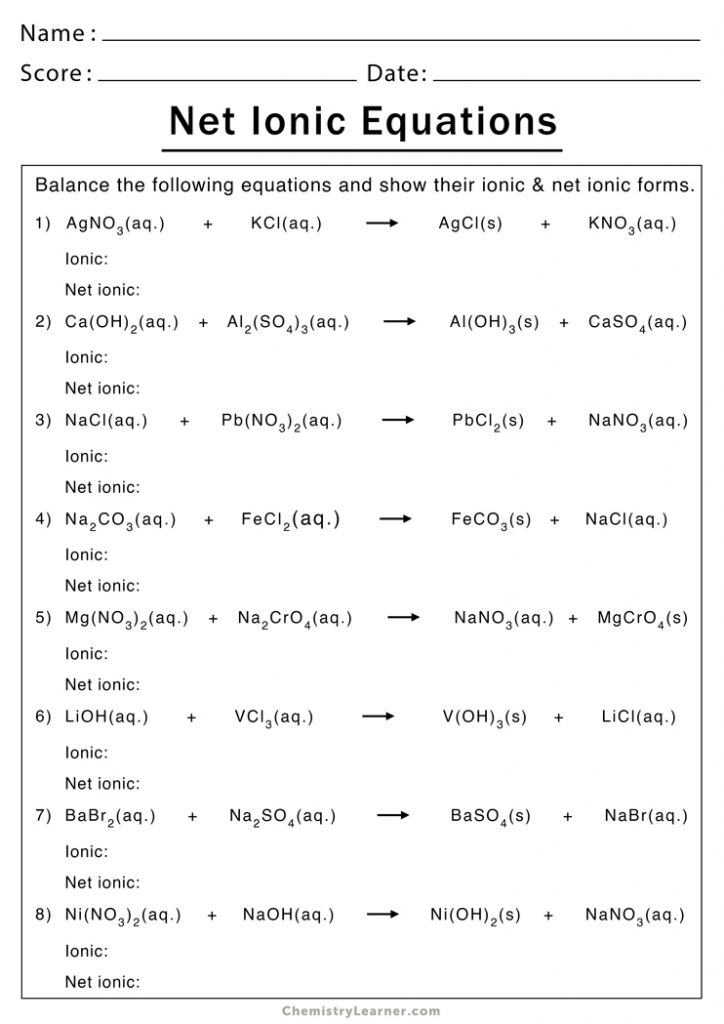
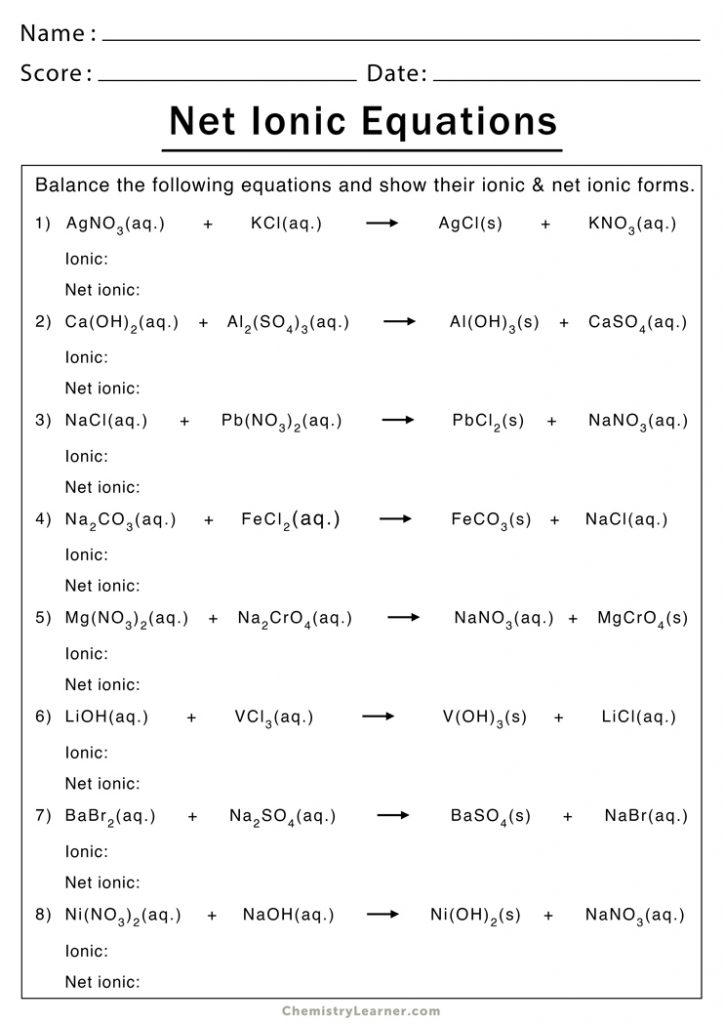
The world of chemistry can often seem daunting, with its myriad of complex reactions and equations. However, one fascinating and foundational concept in chemistry is the ionic equation. This guide aims to demystify ionic equations by providing a comprehensive worksheet guide that helps you understand, practice, and master them. Whether you're a high school student, a college undergrad, or someone simply curious about chemistry, this post will guide you through the intricacies of ionic equations with clear explanations and practical examples.
What Are Ionic Equations?
At their core, ionic equations illustrate chemical reactions where ions are either formed or involved. Unlike molecular equations, which show reactants and products in their molecular forms, ionic equations focus on the ions themselves, providing insight into the actual processes occurring at the ionic level. Here are the key elements:
- Formation of Ions: When substances dissolve in water, they often break apart into their constituent ions.
- Precipitation Reactions: Where ions in solution combine to form a solid.
- Acid-Base Reactions: Where hydrogen ions (H+) or hydroxide ions (OH-) are transferred.
- Net Ionic Equations: Only the species that actually change during the reaction are shown.
Understanding Ionic Dissociation

Before diving into equations, it’s crucial to understand how ionic compounds dissolve:
- Soluble salts dissociate completely into ions in water.
- Insoluble salts don’t dissociate; they form a precipitate instead.
- Acids and bases dissociate based on their strength; strong acids/bases dissociate completely, whereas weak ones do not.
Step-by-Step Guide to Writing Ionic Equations

Here’s how you can approach writing ionic equations:
1. Identify the Type of Reaction

Understanding if it’s a precipitation, acid-base, or another type of reaction will guide your approach. For example, precipitation reactions involve the formation of an insoluble product.
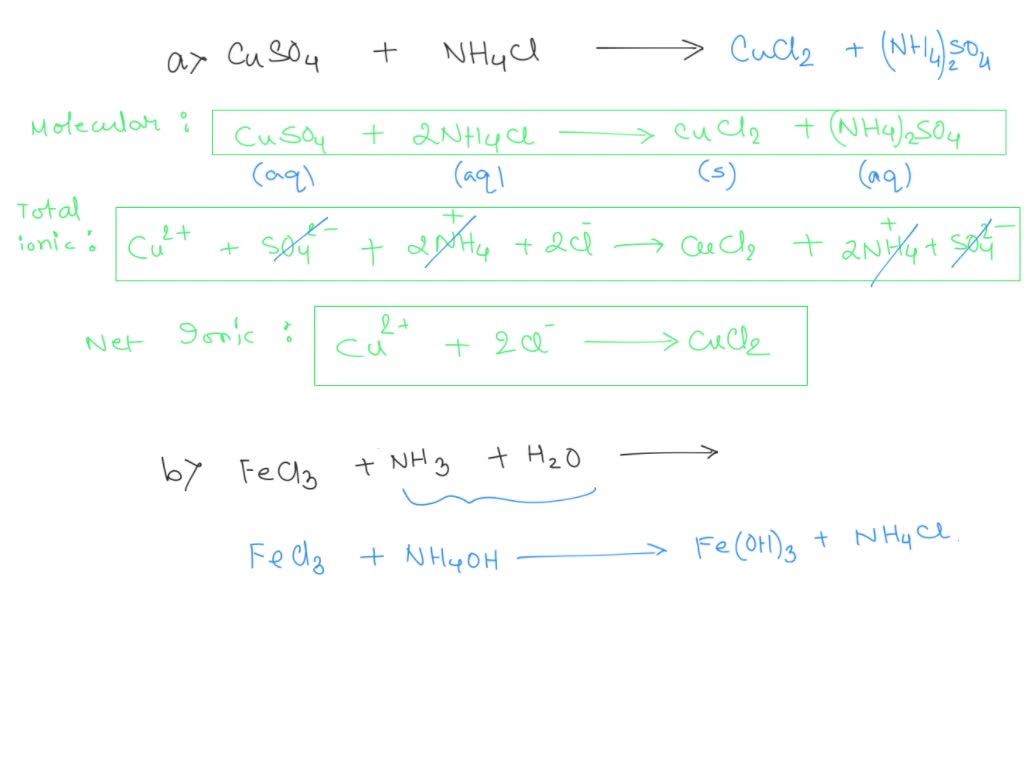
2. Write the Molecular Equation
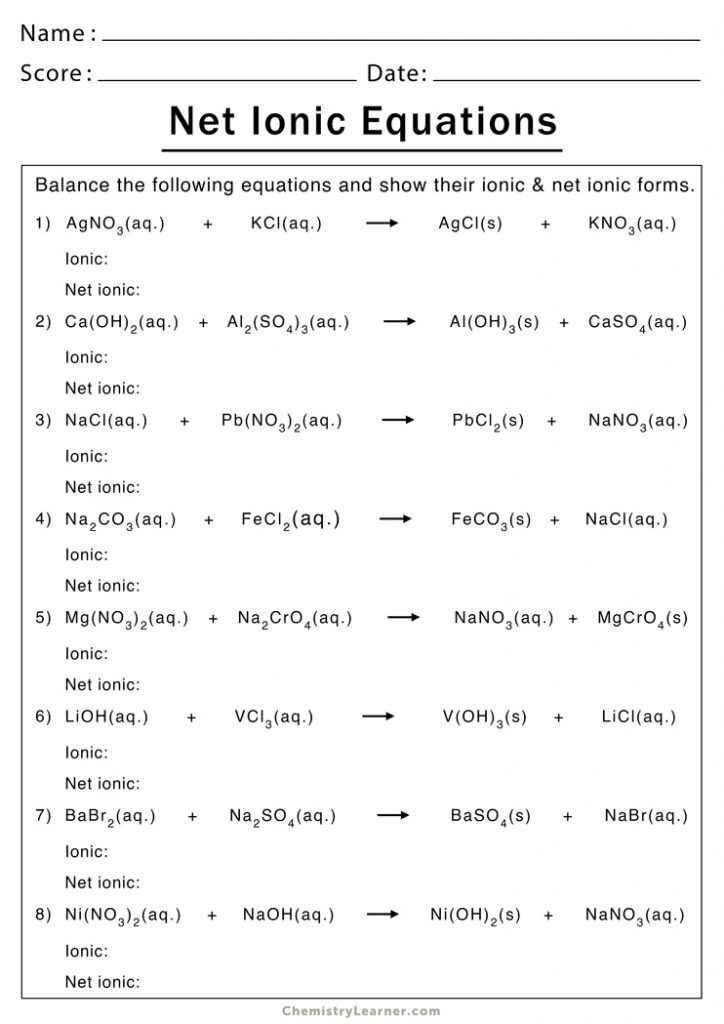
Start by writing down the chemical reaction in its molecular form. Here’s an example of a precipitation reaction:
AgNO3 (aq) + NaCl (aq) → AgCl (s) + NaNO3 (aq)
3. Break into Ions

Next, dissociate all soluble compounds into their respective ions:
Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) → AgCl(s) + Na+(aq) + NO3-(aq)
4. Identify Spectator Ions

Spectator ions are present in the solution but do not participate in the reaction. Here, Na+ and NO3- are spectator ions.
5. Write the Net Ionic Equation

Exclude the spectator ions to show only the ions that change:
Ag+(aq) + Cl-(aq) → AgCl(s)
⚗️ Note: Make sure to balance the charges in the net ionic equation. Ag+ and Cl- combine to form neutral AgCl, for example.
6. Practice with Common Reactions

Here are some common reactions for you to practice:
- Silver nitrate with Sodium Chloride:
- Potassium Iodide with Lead Nitrate:
- Sodium Hydroxide with Magnesium Sulfate:
Common Mistakes to Avoid
- Forgetting to Dissociate Soluble Compounds: Soluble compounds must be broken into ions.
- Neglecting Charges: Each ion carries a specific charge; neglecting this can lead to unbalanced equations.
- Ignoring Spectator Ions: These ions do not participate in the reaction, but they should not be disregarded entirely.
Worksheet for Practice

To solidify your understanding, here’s a small worksheet for practice:
| Problem | Molecular Equation | Net Ionic Equation |
|---|---|---|
| Silver nitrate with Potassium Chloride | ||
| Barium Chloride with Sodium Sulfate | ||
| Acid-Base Reaction: HCl with NaOH |
⚗️ Note: Check solubility rules to predict precipitates and carefully dissociate strong acids/bases.
Tips for Mastering Ionic Equations

- Use Solubility Rules: These rules help you predict which compounds will precipitate out.
- Understand the Concept of Charge Balance: This ensures the equation is chemically logical.
- Practice Visual Representation: Drawing out ions can help in visualizing the reaction.
By working through these steps and engaging with the practice questions, you'll find that mastering ionic equations is not just about memorization but understanding the fundamental principles of ion behavior in aqueous solutions. This understanding will not only make chemistry more accessible but also ignite a deeper interest in how chemical reactions unfold at the atomic and ionic level. With this guide and practice, you'll be well on your way to mastering one of chemistry's most important tools for understanding reactions.
Why are ionic equations important?

+
Ionic equations provide insight into the actual species involved in a chemical reaction, allowing for a deeper understanding of reaction mechanisms, especially in aqueous solutions.
What’s the difference between molecular and ionic equations?
+
Molecular equations show compounds as whole entities, whereas ionic equations show the dissociated ions involved in the reaction, focusing on what actually changes during the process.
How do I know which ions are spectator ions?
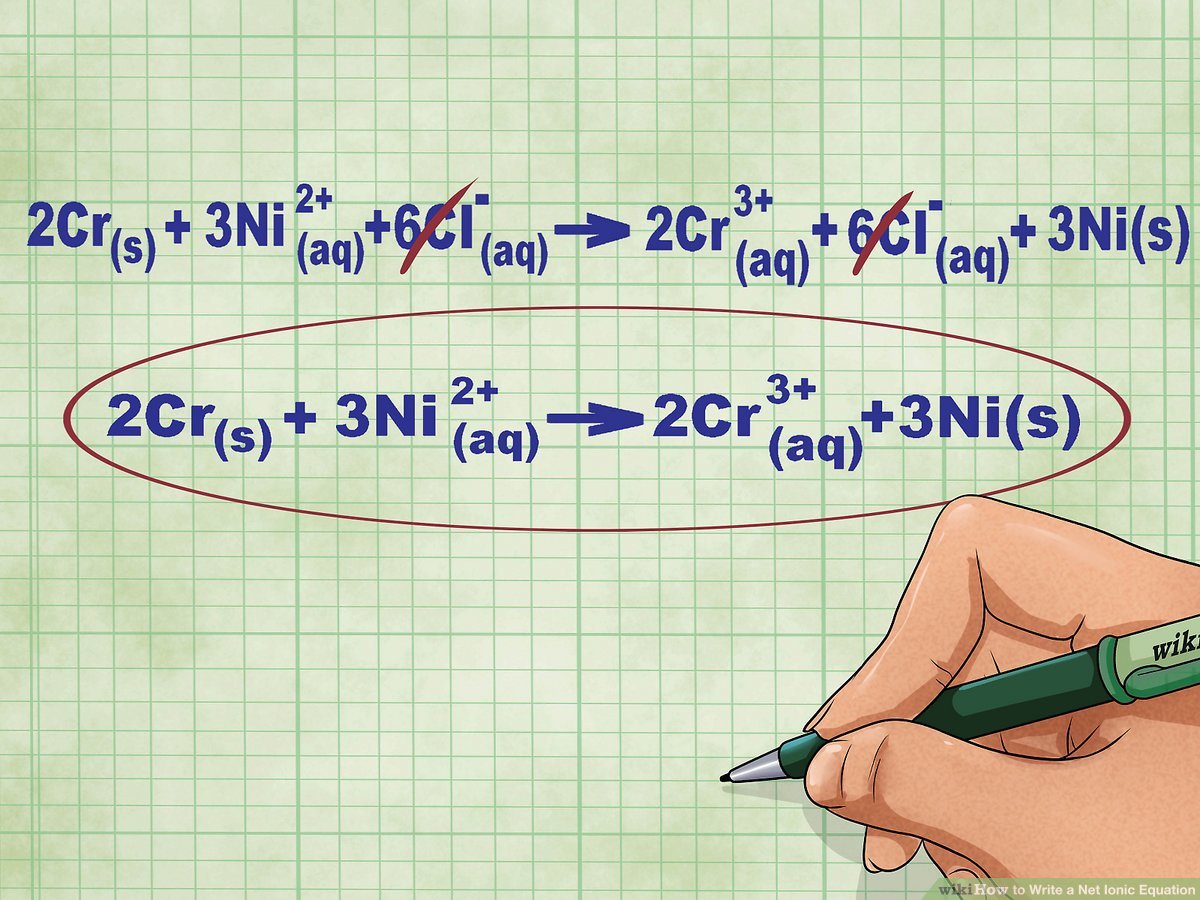
+
Spectator ions remain unchanged on both sides of the equation. They are present but do not participate in the reaction mechanism.