5 Key Answers for Ionic Compounds Naming Worksheet

The naming of ionic compounds is an essential skill in the realm of chemistry. Understanding how to name these compounds not only aids in communication among scientists but also assists in understanding the properties and reactions of the chemicals we deal with daily. In this blog post, we'll delve into five key answers for your Ionic Compounds Naming Worksheet, providing you with the tools to master this aspect of chemical nomenclature.
Understanding Ionic Compounds
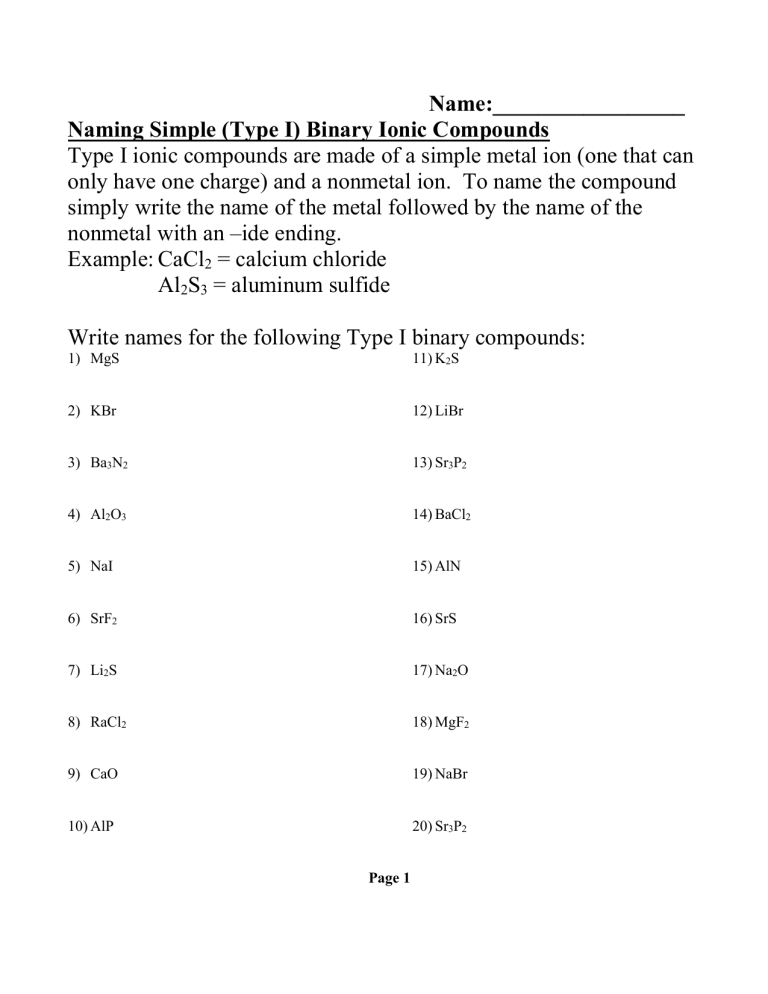

Ionic compounds are formed by the electrostatic attraction between oppositely charged ions. One ion is typically a metal cation, while the other is a non-metal anion. Here’s how to approach their naming:
- Identify the cation - This is generally the metallic element in the formula, losing one or more electrons to become positively charged.
- Identify the anion - Typically a non-metal or polyatomic ion, gaining one or more electrons to become negatively charged.
1. Basic Naming Conventions

The basic naming of ionic compounds follows a straightforward method:
- Write the name of the cation first, as is.
- Follow with the name of the anion, ending in "-ide" if it's a single atom anion, or using its name for polyatomic ions.
For example:
- NaCl = Sodium Chloride
- Ca(OH)2 = Calcium Hydroxide
⚗️ Note: Keep in mind that polyatomic anions have specific names like nitrate (NO3-), sulfate (SO42-), and carbonate (CO32-).
2. Dealing with Variable Charges
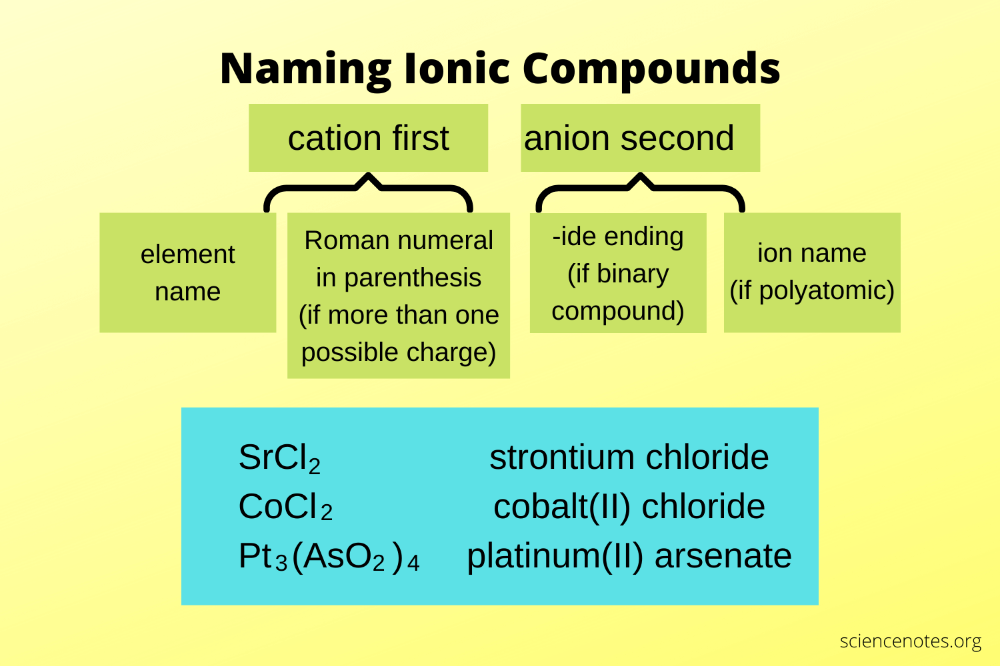
When metals can form multiple ions with different charges, you need to indicate which ion is being used:
- Use Roman numerals in parentheses after the metal name to specify the charge. This is known as the Stock System.
Examples:
- FeCl3 = Iron(III) Chloride
- Cu2O = Copper(I) Oxide
⚗️ Note: You can also use the old naming system with -ic for higher charge and -ous for lower charge, like Ferric Chloride for FeCl3 and Ferrous Chloride for FeCl2.
3. Naming Compounds with Hydrated Ions

When a compound contains water within its crystal structure, the water molecules are called waters of hydration, and you include them in the name:
- Write the name of the compound as usual, followed by "dot," then the number of waters, and "hydrate."
For example:
- CuSO4·5H2O = Copper(II) Sulfate Pentahydrate
4. Complex Ions

For compounds involving complex ions, the following rules apply:
- Start with the cation, then the complex anion or complex cation followed by the anions or ligands. Enclose the complex ion in square brackets in the name.
Example:
- K[Fe(CN)6] = Potassium Hexacyanoferrate(II)
5. Binary Compounds with Nonmetals

When dealing with binary compounds where both elements are nonmetals, use the Greek prefixes to indicate the number of atoms:
| Number | Prefix |
|---|---|
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |

Examples:
- PCl5 = Phosphorus Pentachloride
- N2O = Dinitrogen Monoxide
In this journey through the naming of ionic compounds, we’ve covered several essential points that are critical for anyone involved in chemistry, from students to seasoned professionals. By understanding how to name these compounds, we not only clarify the substances we’re dealing with but also facilitate better communication within the scientific community. Each rule and example has been designed to give you a clearer picture of how to approach this topic, whether you’re dealing with simple binary compounds or more complex ions with variable charges or hydration states.
To summarize, we’ve explored:
- Basic Naming Conventions: How to name compounds by simply identifying the cation and anion, with straightforward rules for single atom anions and polyatomic ions.
- Dealing with Variable Charges: The use of Roman numerals or the older naming system to indicate the charge of metals that can form multiple ions.
- Hydrated Ions: Naming compounds that contain water within their crystal structure, using waters of hydration in the name.
- Complex Ions: The rules for naming compounds with complex ions, ensuring the complex part is named accordingly.
- Binary Compounds with Nonmetals: Using Greek prefixes to signify the number of atoms in compounds where both elements are nonmetals.
The key takeaway is that while the naming of ionic compounds can seem complex, following these systematic rules will enable you to correctly name most compounds you’ll encounter. Furthermore, this understanding can serve as a springboard for more advanced chemical naming conventions, such as organic compounds or coordination compounds.
What are the differences between ionic and covalent compound naming?

+
Ionic compounds focus on the electrostatic attraction between ions, often metals with non-metals or polyatomic ions. Naming typically involves the metal name followed by the non-metal with an “-ide” suffix. Covalent compounds, on the other hand, involve sharing of electrons and use Greek prefixes to denote the number of atoms of each element involved.
How do I know if a metal has a variable charge?

+
You can determine if a metal has a variable charge by its position in the periodic table. Transition metals, particularly those in groups 3-12, often have variable charges. Also, metals like iron, copper, and lead are known for having multiple charges. A periodic table with ion charges or a reliable list of common charges can be helpful.
Is there a limit to how many waters of hydration a compound can have?

+
The number of waters of hydration a compound can have isn’t strictly limited but is dependent on the stability and structure of the compound. Some compounds might take in a few water molecules, while others might absorb many, like Copper(II) Sulfate which can form pentahydrate, decahydrate, and even higher hydrates under different conditions.
How do you name compounds with polyatomic ions?

+
Naming compounds with polyatomic ions is similar to naming simple ionic compounds. You state the name of the metal cation first, followed by the name of the polyatomic ion, which you use as is, without modifying its name.
What are coordination compounds, and how are they named?

+
Coordination compounds involve a central metal ion surrounded by ligands. Naming these compounds is more complex and involves the cation, the name of the complex ion, the central metal with its oxidation state, and the ligands named with appropriate prefixes to indicate the number. The rules can get intricate, but the basic structure follows the name of the cation first, then the complex anion.