5 Essential Steps to Understanding Ionic Bonds

Understanding how atoms bond together is fundamental in chemistry. This comprehensive guide walks you through the five essential steps to grasp the concept of ionic bonding, providing you with a robust foundation in one of the key interactions between atoms. By diving deep into the nature of ions and their interaction, you'll not only learn about ionic bonds but also enhance your understanding of chemical reactivity and structure.
Step 1: Electron Configuration

The first step in understanding ionic bonds is to get familiar with electron configurations. Atoms aim for stability by having a full outer shell of electrons. The valence electrons, located in the outermost shell, play a pivotal role:
- Noble Gas Configuration: Elements like helium, neon, and argon have fully occupied outermost shells, rendering them stable and non-reactive.
- Electron Octet: Atoms seek to achieve a noble gas configuration, either by gaining or losing electrons.
🧠 Note: Understanding electron configuration helps predict an atom's tendency to form bonds.
Step 2: Ion Formation

When atoms gain or lose electrons to reach a stable configuration, they become ions:
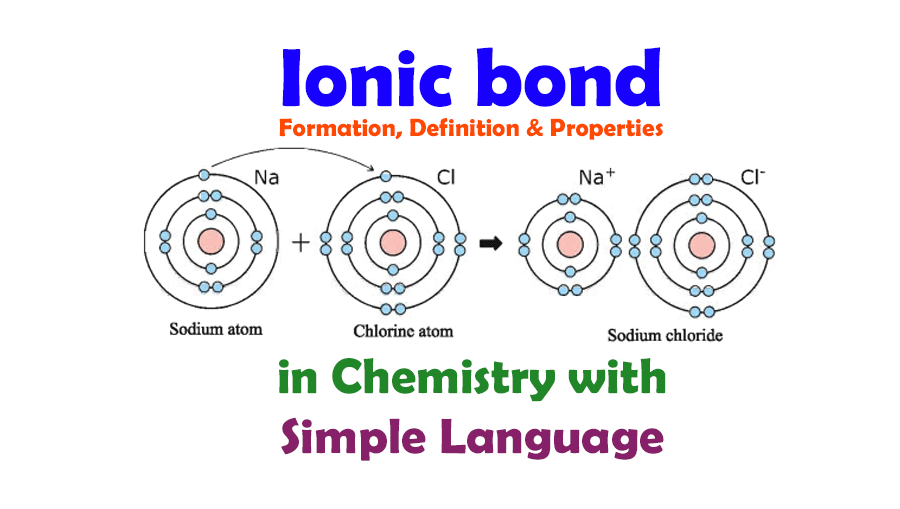
- Cations: Positively charged ions formed when atoms lose electrons. For example, sodium loses one electron to become Na+.
- Anions: Negatively charged ions formed when atoms gain electrons. For instance, chlorine gains one electron to become Cl-.
Here's a simple table to illustrate the ion formation process:
| Atom | Electrons Lost/Gained | Ion Formed | Charge |
|---|---|---|---|
| Sodium (Na) | 1 lost | Na+ | +1 |
| Chlorine (Cl) | 1 gained | Cl- | -1 |

Step 3: Ionic Bonding

Once ions are formed, they interact through electrostatic forces. Here’s how an ionic bond is established:
- Ionic bonds result from the attraction between positively charged cations and negatively charged anions.
- These bonds create a stable compound by balancing out the charges.
Take for example sodium chloride (NaCl): Sodium (Na) loses an electron to become Na+, and chlorine (Cl) gains an electron to become Cl-. The electrostatic attraction between these ions forms an ionic bond, leading to the formation of NaCl.
Step 4: Lattice Energy
Ionic compounds are structured in a crystal lattice. Lattice energy is key here:
- The lattice energy is the energy required to separate one mole of a solid ionic compound into gaseous ions.
- The greater the lattice energy, the stronger the ionic bond.
- Factors affecting lattice energy include ion charge, ionic radius, and bond length.
Step 5: Properties of Ionic Compounds

Ionic bonds significantly influence the properties of ionic compounds:
- High Melting and Boiling Points: Due to strong electrostatic forces, ionic compounds require high energy to break their lattice structure.
- Solubility: They often dissolve in polar solvents like water, which can disrupt the ionic lattice.
- Electrical Conductivity: When molten or dissolved, ionic compounds conduct electricity due to the presence of free ions.
In summary, the journey of understanding ionic bonds encompasses recognizing the electron configuration, ion formation, the nature of ionic bonding, lattice energy, and the unique properties of ionic compounds. This knowledge not only broadens your understanding of how elements combine but also unlocks the door to predicting reactions and exploring the physical and chemical behavior of substances.
What’s the difference between an ionic and a covalent bond?

+
Ionic bonds involve the transfer of electrons between atoms, resulting in a strong electrostatic attraction between oppositely charged ions. Conversely, covalent bonds occur when atoms share electrons to achieve a stable electron configuration.
Why do ionic compounds dissolve in water?

+
Water molecules, being polar, can pull the ionic lattice apart by surrounding and isolating the ions, which leads to their dissolution. The interaction between the water molecules and ions helps stabilize the separated ions in solution.
How does lattice energy affect the stability of ionic compounds?

+
Higher lattice energy indicates stronger bonds between the ions in the crystal lattice, leading to more stable ionic compounds. This is due to the higher energy requirement for breaking these bonds apart, making the compound more stable in its solid form.



