5 Key Answers for Ionic Bonds Worksheet

Ionic bonds are fundamental in the realm of chemistry, providing the backbone for numerous chemical compounds that we encounter daily. Understanding how these bonds form and their characteristics can offer profound insights into the behavior and properties of materials. Today, we're going to delve into the world of ionic bonding through a practical guide: providing you with five key answers for your ionic bonds worksheet.
Ionic Bonding Basics

Ionic bonding occurs when one atom donates electrons to another, leading to the formation of ions with opposite charges that attract each other. Here are the fundamental concepts to grasp:
- Valence Electrons: The outermost electrons in an atom, which participate in chemical bonding.
- Electronegativity: A measure of an atom's ability to attract and hold onto electrons. Higher electronegativity leads to an atom gaining electrons, becoming negatively charged (anion).
- Electropositive: Elements with low electronegativity tend to lose electrons, forming positively charged ions (cations).
Key Answers for Ionic Bonds Worksheet

1. What Determines if an Ionic Bond Will Form?

An ionic bond forms when the electronegativity difference between two atoms is significant. Here are the key factors:
- The difference in electronegativity should be at least 1.7 for ionic bonding to occur.
- The ability of one element to lose electrons (metals) and another to gain electrons (nonmetals or metalloids).
2. How Do Ions Form?

Ions are formed through:
- Cation Formation: An atom loses one or more electrons, leading to a positive charge. This is common in metals, like sodium (Na+).
- Anion Formation: An atom gains one or more electrons, resulting in a negative charge. Nonmetals, such as chlorine (Cl-), often form anions.
3. Predicting Compound Formation

When predicting the formation of ionic compounds:
- Look at the position of elements on the periodic table. Elements towards the left tend to lose electrons, forming cations; those on the right gain electrons to become anions.
- Check the valency, which indicates how many electrons an atom will lose or gain to achieve a stable electron configuration (usually a full outer shell).
- The compound formed must ensure charge balance; the total positive charge equals the total negative charge.
💡 Note: For compounds with polyatomic ions, like SO42-, remember that the entire ion carries the charge, not just the sulfur atom.
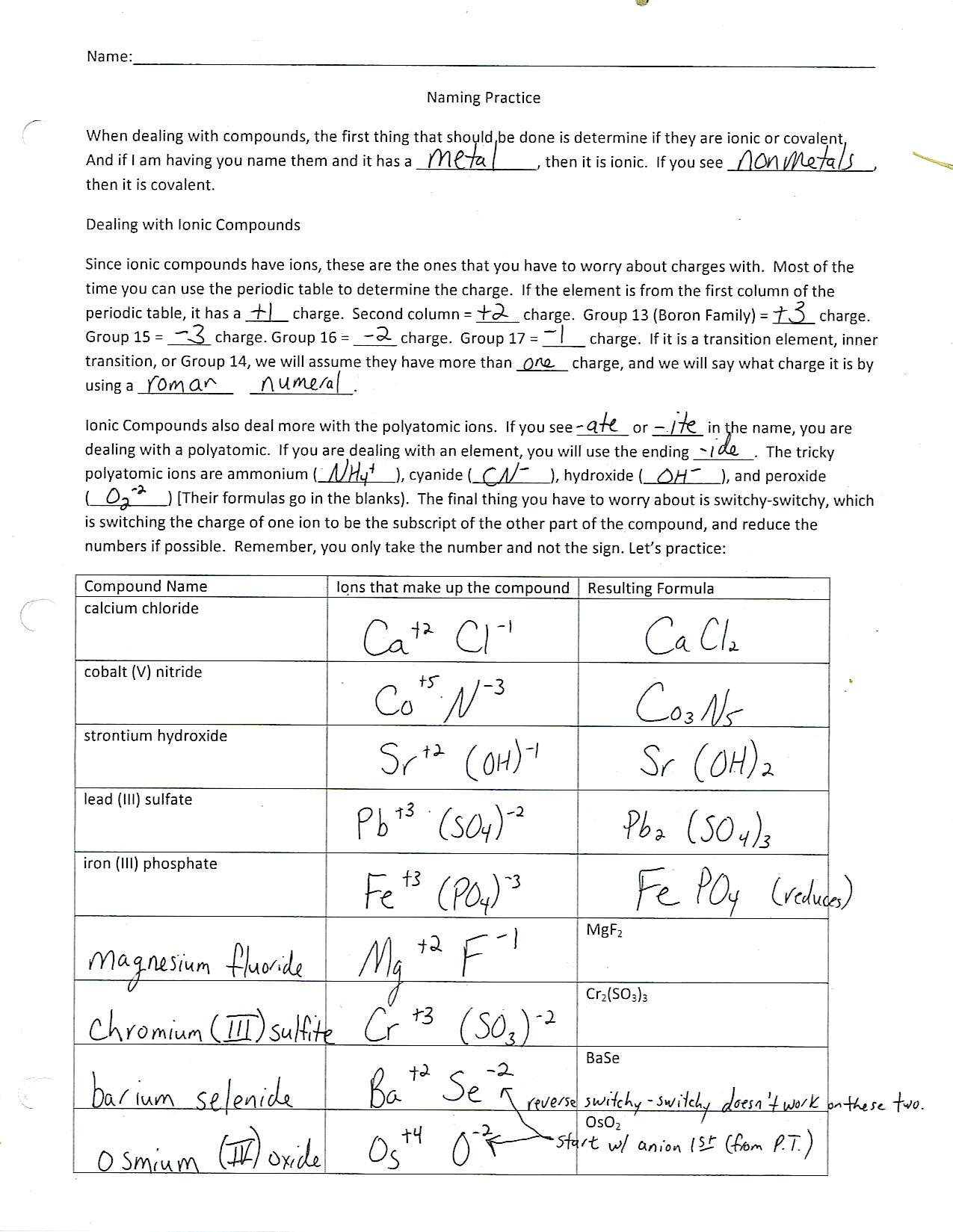
4. Naming Ionic Compounds

Naming ionic compounds involves:
- The cation’s name remains unchanged from its elemental form (e.g., sodium, potassium).
- The anion’s name changes to end in -ide (e.g., chloride, oxide). For polyatomic ions, the name stays as it is (e.g., sulfate, nitrate).
- If the metal can form more than one cation (like transition metals), Roman numerals indicate the charge (e.g., Fe3+ becomes iron(III)).
5. Ionic Bonding and Physical Properties
Ionic bonding influences the physical properties of compounds:
- High Melting Points: Due to strong electrostatic forces between ions, ionic compounds generally have high melting points.
- Brittleness: Ionic solids are brittle because when stress is applied, like layers can shift, causing ions with like charges to come into contact and repel.
- Solubility: Ionic compounds often dissolve in polar solvents like water because of the interaction between the solvent’s partial charges and the ions.
- Electrical Conductivity: While solid ionic compounds do not conduct electricity, they do so when melted or dissolved in water due to the mobility of free ions.
💡 Note: The presence of water molecules can alter the solubility and electrical conductivity of ionic compounds. Water's polarity plays a significant role in this process.
In wrapping up our journey through the fascinating world of ionic bonds, we’ve covered the core principles necessary for tackling any ionic bonds worksheet. From understanding how these bonds form to their influence on the physical properties of compounds, you’re now better equipped to comprehend and explain the dynamic interactions within chemical systems. Remember, ionic bonds not only tell a story of electrons but also provide a foundational understanding of material behavior in various conditions, making chemistry not just a science but an art of predicting and explaining the very nature of substances around us.
The insights gained here can be applied to everyday scenarios, from the hardness of minerals to the conductivity of electrolytes in batteries. This understanding is not just for the classroom; it’s a fundamental part of how we interact with and utilize the world around us.
Why do ionic compounds dissolve in water?

+
Water is a polar solvent, meaning it has partial charges (slightly positive hydrogen atoms and slightly negative oxygen atoms). These partial charges interact with the ions in an ionic compound, pulling them apart and surrounding them with water molecules, leading to the dissolution of the compound.
How can I predict the charge on an ion?

+
The charge on an ion can often be predicted from its position in the periodic table. Elements on the left side (groups 1 and 2) lose electrons to become cations with charges of +1 and +2, respectively. Elements on the right (groups 16 and 17) gain electrons to become anions with charges of -2 and -1, respectively. Transition metals can vary but commonly form +2 or +3 ions.
Can ionic bonds be broken?

+
Yes, ionic bonds can be broken, but doing so requires significant energy due to the strong electrostatic attractions between ions. This typically happens at high temperatures (melting) or in solutions (dissolving), where ions become free to move, effectively ‘breaking’ the bond.