5 Ionic Bonding Tips
Understanding Ionic Bonding

Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions. It is a crucial concept in chemistry, and understanding it is essential for students and professionals alike. In this article, we will discuss the basics of ionic bonding and provide five tips to help you master this concept.
What is Ionic Bonding?

Ionic bonding occurs when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. The atom that loses electrons becomes a positively charged ion, known as a cation, while the atom that gains electrons becomes a negatively charged ion, known as an anion. The electrostatic attraction between the cation and anion holds them together, forming a strong chemical bond.
Key Concepts in Ionic Bonding
Before we dive into the tips, it’s essential to understand some key concepts in ionic bonding. These include: * Electronegativity: The ability of an atom to attract electrons towards itself. * Ionization energy: The energy required to remove an electron from an atom. * Electron affinity: The energy released when an electron is added to an atom. * Lattice energy: The energy released when ions come together to form a crystal lattice.
5 Ionic Bonding Tips
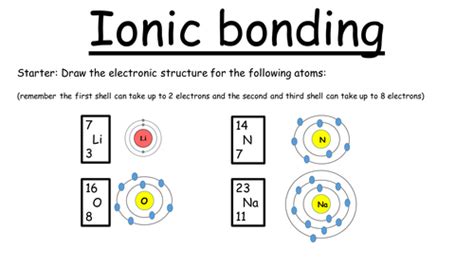
Here are five tips to help you master ionic bonding: * Tip 1: Understand the Electron Configuration: To form an ionic bond, atoms must have a full outer energy level. This means that the atom must have a noble gas configuration. By understanding the electron configuration of atoms, you can predict which atoms are likely to form ionic bonds. * Tip 2: Identify the Cation and Anion: In an ionic compound, one atom loses electrons to form a cation, while another atom gains electrons to form an anion. Identifying the cation and anion is crucial in understanding the ionic bonding process. * Tip 3: Determine the Charge on the Ions: The charge on the ions is critical in ionic bonding. The charge on the cation is positive, while the charge on the anion is negative. The magnitude of the charge depends on the number of electrons lost or gained. * Tip 4: Use the Octet Rule: The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level with eight electrons. This rule is helpful in predicting the formation of ionic bonds. * Tip 5: Consider the Lattice Energy: Lattice energy is the energy released when ions come together to form a crystal lattice. It is an essential factor in determining the stability of an ionic compound.
Examples of Ionic Compounds

Here are some examples of ionic compounds: * Sodium chloride (NaCl) * Calcium carbonate (CaCO3) * Aluminum oxide (Al2O3) These compounds are formed through the transfer of electrons between atoms, resulting in the formation of ions with opposite charges.
Factors Affecting Ionic Bonding

Several factors can affect the formation and strength of ionic bonds. These include: * Size of the ions: The size of the ions can affect the lattice energy and the stability of the ionic compound. * Charge of the ions: The charge on the ions can affect the electrostatic attraction between the cation and anion. * Polarity of the ions: The polarity of the ions can affect the distribution of electrons and the strength of the ionic bond.
| Ion | Charge | Size |
|---|---|---|
| Sodium (Na+) | +1 | Small |
| Chloride (Cl-) | -1 | Large |
| Calcium (Ca2+) | +2 | Medium |

📝 Note: Understanding the factors that affect ionic bonding is crucial in predicting the formation and stability of ionic compounds.
To summarize, ionic bonding is a complex concept that involves the transfer of electrons between atoms, resulting in the formation of ions with opposite charges. By understanding the key concepts, tips, and factors that affect ionic bonding, you can master this essential concept in chemistry. Remember to apply the octet rule, identify the cation and anion, and consider the lattice energy when predicting the formation of ionic compounds.
What is the main difference between ionic and covalent bonding?

+
The main difference between ionic and covalent bonding is the transfer of electrons. In ionic bonding, electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. In covalent bonding, electrons are shared between atoms.
How do you predict the formation of ionic compounds?

+
To predict the formation of ionic compounds, you need to consider the electron configuration of the atoms involved, the charge on the ions, and the lattice energy. You can also apply the octet rule and identify the cation and anion.
What is the importance of lattice energy in ionic bonding?

+
Lattice energy is essential in ionic bonding because it determines the stability of the ionic compound. The lattice energy is the energy released when ions come together to form a crystal lattice. A higher lattice energy indicates a more stable ionic compound.



