Ionic Bonding Lewis Dot Structures: Master with This Worksheet

Ionic bonding is one of the fundamental concepts in chemistry, allowing us to understand how atoms interact to form compounds. It's especially relevant when learning about chemical stability and the octet rule. Mastering ionic bonding through Lewis dot structures provides a visual and conceptual framework for understanding this crucial interaction. Whether you're a student preparing for exams or a chemistry enthusiast, this detailed guide will walk you through the process using structured worksheets, practical examples, and key principles.
Understanding Ionic Bonding

Ionic bonding occurs when there’s a complete transfer of electrons from one atom to another, resulting in the formation of positively charged cations and negatively charged anions. This transfer happens to achieve electron configurations similar to the noble gases, which are extremely stable due to their full valence electron shells.
- Metal atoms tend to lose electrons, becoming cations.
- Non-metal atoms gain these electrons, becoming anions.
What are Lewis Dot Structures?

Lewis dot structures, or electron dot diagrams, are diagrams that show the bonding between atoms of a molecule, and the lone pairs of electrons that may exist. They are invaluable tools for visualizing:
- The number of valence electrons each atom has.
- How these electrons are shared or transferred during bond formation.
Steps to Draw Lewis Dot Structures for Ionic Compounds

Here’s a systematic approach to draw Lewis dot structures for ionic compounds:
- Identify the Atoms Involved: Note which elements are present in the compound.
- Determine Valence Electrons: Count the valence electrons for each atom. For cations, subtract electrons corresponding to the positive charge, and for anions, add electrons for the negative charge.
- Create the Structure: Place the cation on the left and the anion(s) on the right. Use dots to represent the valence electrons.
- The cation will usually have all its valence electrons removed.
- The anion will have electrons added to achieve a full octet.
- Transfer Electrons: Draw the transfer of electrons from the cation to the anion using arrows or just place the electrons where they belong in the final structure.
- Check Your Work: Ensure both atoms have achieved noble gas configurations (full octets).
Practical Examples

Let’s walk through some examples to illustrate the process:
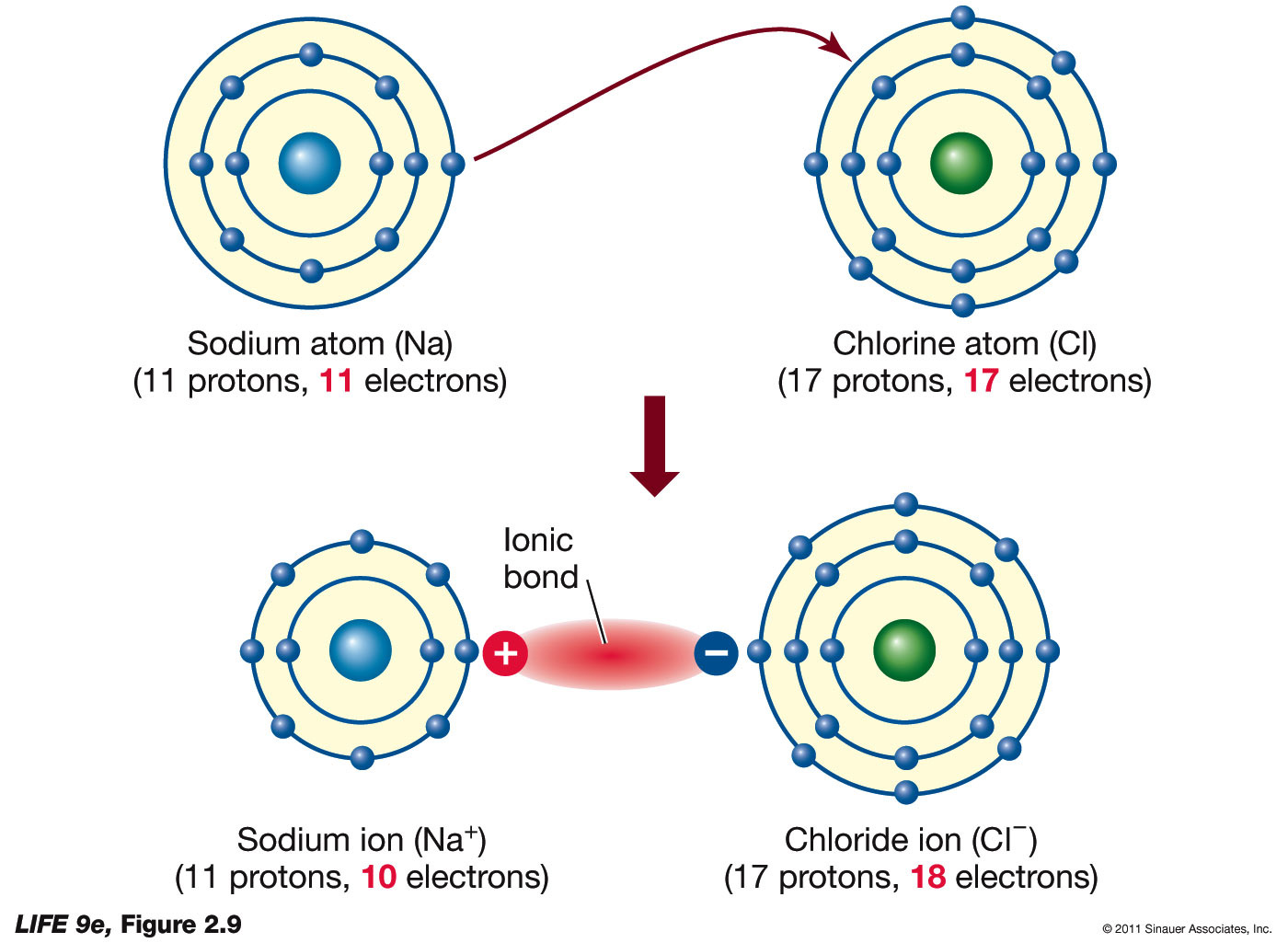
Sodium Chloride (NaCl)

| Step | Description |
|---|---|
| Identify Atoms | Sodium (Na) and Chlorine (Cl) |
| Valence Electrons | Na: 1 valence electron, Cl: 7 valence electrons |
| Create Structure | Na+: no electrons, Cl-:·Cl· (dots to the left and right) |
| Transfer | Na loses 1 electron to Cl |
| Final Structure | Na+Cl- or [Na]+[Cl]- (showing noble gas configurations) |

Magnesium Oxide (MgO)
Follow the same steps, adjusting for magnesium losing two electrons and oxygen gaining two:
Worksheet for Practice

Here are some compounds to practice drawing Lewis dot structures for ionic bonding:
- Calcium Bromide (CaBr2)
- Aluminum Nitride (AlN)
- Potassium Sulfide (K2S)
Try drawing these structures, focusing on:
- Balancing the charges to ensure neutrality.
- Ensuring each atom has achieved a stable octet (or in some cases, an expanded octet).
🚀 Note: When dealing with polyatomic ions, treat them as a single unit that forms a part of the ionic compound. For example, in potassium sulfate (K2SO4), the sulfate ion (SO42-) is a polyatomic ion.
⚠️ Note: While drawing Lewis structures for ionic compounds, the main focus is on electron transfer, not electron sharing, which is more typical for covalent bonding.
The process of understanding and drawing Lewis dot structures for ionic compounds not only deepens your knowledge of chemical interactions but also aids in predicting and understanding the properties of different compounds. As you practice, you'll find that patterns emerge, making the task of predicting and drawing ionic bond structures increasingly intuitive.
Through this guide, you've learned the steps for creating Lewis dot structures for ionic compounds, enhancing your comprehension of how atoms achieve stability through ionic bonding. Remember that like any skill, proficiency in drawing these structures comes with practice, which helps to internalize the principles of electron transfer and octet rule stability.
What is the difference between ionic and covalent bonding?

+
Ionic bonding involves the transfer of electrons between atoms, resulting in ions that attract each other due to opposite charges. Covalent bonding, on the other hand, involves the sharing of electrons between atoms to achieve a full octet.
Why do metals form cations and non-metals form anions?

+
Metals, which are on the left of the periodic table, have fewer electrons in their outer shell, making it easier for them to lose electrons and achieve a stable configuration. Non-metals, with more electrons in their outer shell, prefer to gain electrons to fill their octet and become negatively charged anions.
Can an element form both ionic and covalent bonds?

+
Yes, some elements can form both types of bonds depending on the context. For example, hydrogen can form a covalent bond with itself (H2) or form an ionic bond with sodium in sodium hydride (NaH).