5 Essential Tips for Ionic Bonding Worksheets

Mastering ionic bonding can be a challenging yet fascinating part of learning chemistry, especially when you're delving into the world of compounds, elements, and their interactions. To help students grasp this concept effectively, educators often employ Ionic Bonding Worksheets. Here, we'll explore 5 essential tips that not only aid in the creation of such worksheets but also enhance the learning experience, ensuring both students and teachers can approach ionic bonding with confidence.
1. Focus on Visual Representation

- Use Diagrams: Ionic bonds occur when one atom gives away electrons to another to achieve a full valence shell. Diagrams illustrating this electron transfer are invaluable. Include:
- Bohr Models to represent atoms before and after bonding.
- Electron Dot Diagrams to show which atoms will likely form ionic bonds.
🧑🔬 Note: Visual representations should always be labeled accurately to ensure clarity. Complex diagrams might need a key to guide students.

2. Incorporate Interactive Questions

- Engage Through Inquiry: Ask questions that require students to think critically about ionic bonds:
- “Why do sodium and chlorine form a bond?”
- “What happens when a metal atom loses electrons?”
- Include fill-in-the-blanks, multiple-choice questions, or true/false statements that foster active learning.
💡 Note: Answers should not be given on the same worksheet to promote independent thinking and later teacher-student interaction.
3. Use Real-World Examples

- Application in Everyday Life: Relate ionic bonding to things students encounter in their daily life:
- Table salt (NaCl) as an example of an ionic compound.
- Ceramics and their ionic components.
🌍 Note: Real-world applications help students understand why ionic bonds are relevant beyond the classroom.
4. Provide Step-by-Step Exercises

- Worksheet Structure: Break down the process of understanding ionic bonding into manageable steps:
- Identify the elements and their charges.
- Determine which atoms will lose or gain electrons.
- Draw or represent the electron transfer.
- Balance the charges to form an ionic compound.
- Complete the molecular formula.
| Step | Action |
|---|---|
| 1 | Identify elements (Na and Cl) |
| 2 | Sodium loses one electron, chlorine gains one |
| 3 | Draw transfer of electrons |
| 4 | Na+ + Cl- = NaCl |
| 5 | Formula is NaCl |

📝 Note: Step-by-step exercises help students visualize and understand each part of the process of ionic bonding.
5. Tailor to Skill Levels
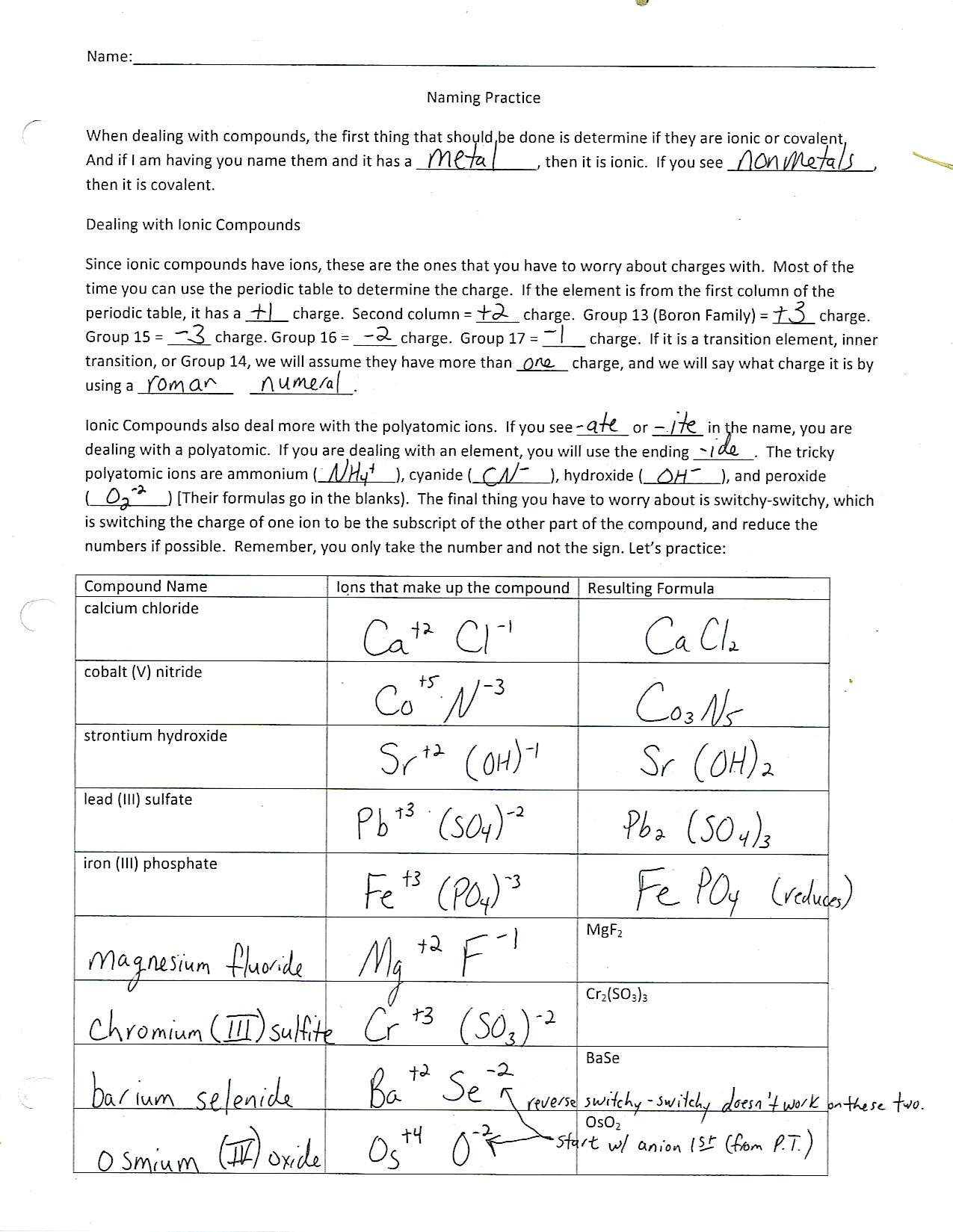
- Differentiation: Adjust the complexity of worksheets:
- Begin with simple compounds and increase complexity.
- Introduce polyatomic ions for more advanced learners.
- Include cross-curricular questions for a holistic learning approach.
- Ensure that there is a clear progression in the worksheet from basic to advanced topics to cater to students’ varied capabilities.
👥 Note: A one-size-fits-all approach rarely works in education. Tailoring content allows all students to grow at their own pace.
The art of creating ionic bonding worksheets lies in a combination of clear instruction, engaging material, and tailored learning experiences. From visual aids to real-world applications, incorporating these tips ensures that students not only learn the mechanics of ionic bonding but also appreciate its significance. As educators, our goal is to lay the foundation for deeper understanding, curiosity, and a genuine love for chemistry.
What is the main difference between ionic and covalent bonding?

+
Ionic bonding involves the transfer of electrons from one atom to another, typically between a metal and a non-metal, creating charged ions. In contrast, covalent bonding involves the sharing of electrons between atoms, typically between non-metals.
How can I determine if an atom will lose or gain electrons?

+
Atoms lose electrons to achieve a full outer electron shell, particularly when they have 1-3 valence electrons (like sodium with one valence electron). Atoms gain electrons if they need 1-3 electrons to complete their valence shell (like chlorine with seven valence electrons).
Why is visual representation important in ionic bonding?

+
Visual representations help students visualize abstract concepts like electron transfer, making it easier to understand the process of ionic bond formation.



