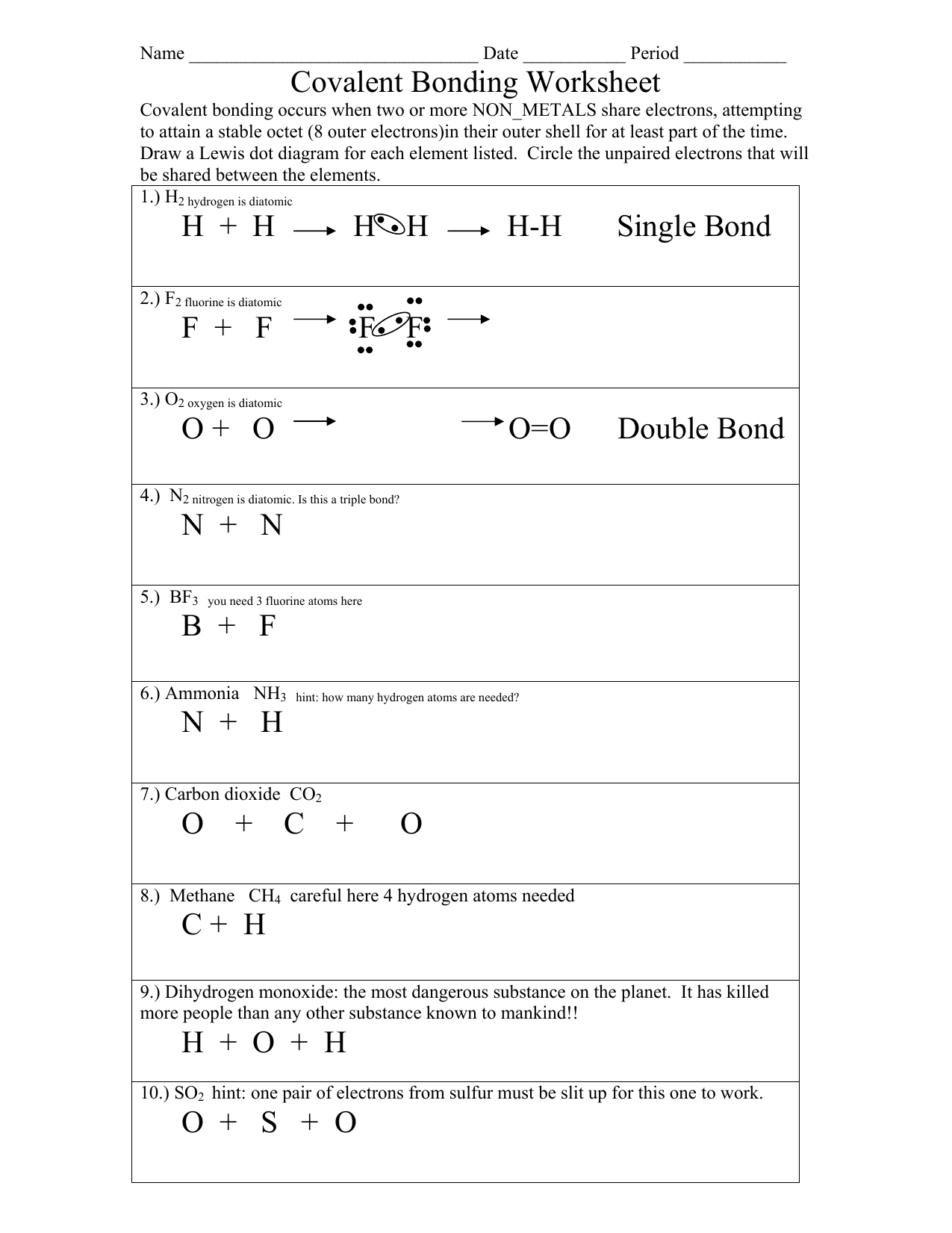
Ionic and Covalent Bonds Color Activity Sheet

Understanding the essence of chemical bonds is fundamental in the study of chemistry. These bonds, primarily ionic and covalent, govern how atoms interact to form compounds, influencing the physical and chemical properties of substances. A visually engaging approach, such as a color activity, can significantly enhance comprehension, making the sometimes abstract concept of chemical bonding more tangible. This activity involves coloring and identifying various compounds as either ionic or covalent, offering students a hands-on learning experience.
Materials Needed

Before diving into the activity, gather these essentials:
- Coloring tools like crayons, markers, or colored pencils
- Ionic and covalent bonds color activity sheet (available from your teacher or online resources)
- A Periodic Table for reference
- Additional notes or textbooks on chemical bonding (optional)
Understanding Ionic and Covalent Bonds

Before we proceed with the activity, let’s clarify what ionic and covalent bonds entail:
- Ionic Bonds: Formed when one atom loses one or more electrons, transferring them to another atom, creating charged ions that attract each other. Here, metals often bond with non-metals.
- Covalent Bonds: Occur when atoms share electrons to achieve a stable electron configuration. Typically, these bonds form between non-metals.
💡 Note: The octet rule plays a significant role in both types of bonds, where atoms tend to achieve an outer electron configuration of 8 to gain stability.
The Coloring Activity

Follow these steps for the ionic and covalent bonds color activity:
Step 1: Identify the Bond Type

Using the Periodic Table, identify the elements in each compound on your activity sheet:
- Mark metals with one color, typically blue or green, to symbolize positive ions.
- Choose another color, often red or pink, for non-metals, signifying negative ions or electron sharing.
Step 2: Color the Compounds

Now, color each compound:
- Use the color for metals to fill in or outline positive ions in ionic compounds.
- For covalent compounds, color both atoms the same non-metal color, or use different shades to indicate electron sharing.
Step 3: Analyze the Coloring
Observe the patterns:
- Ionic compounds should have a clear distinction between positive (metal) and negative (non-metal) ions.
- Covalent compounds will show a blending or sharing of colors, signifying electron sharing.
Table: Examples of Ionic and Covalent Compounds

| Compound | Type of Bond | Description |
|---|---|---|
| NaCl (Sodium Chloride) | Ionic | Na+ donates an electron to Cl-, forming a stable ionic compound. |
| H2O (Water) | Covalent | Hydrogen and oxygen share electrons to form polar covalent bonds. |
| MgO (Magnesium Oxide) | Ionic | Magnesium gives up two electrons to oxygen, creating the Mg2+O2- compound. |
| CO2 (Carbon Dioxide) | Covalent | Double covalent bonds form between carbon and oxygen atoms, sharing electrons. |

Recapping the core insights from this activity, we've explored how atoms bond to achieve stability through ionic or covalent bonding. By color-coding different compounds, students gain a visual understanding of these bonds, distinguishing between metal and non-metal interactions. This not only solidifies the concept of electronegativity, electronegativity, and the Octet Rule but also fosters an appreciation for the periodic table's role in predicting chemical behavior.
What is the difference between ionic and covalent bonds?

+
Ionic bonds involve the complete transfer of electrons between atoms, typically between metals and non-metals, forming ions. Covalent bonds, in contrast, occur when atoms share electrons to fill their outer shells, usually between non-metals or between a metal and a non-metal with low electronegativity difference.
Why do we use colors in this activity?

+
Colors help visualize the type of bond and can serve as an mnemonic device to remember the nature of different compounds. Blue or green often represent metals (positive ions), while red or pink are used for non-metals (negative ions or electron sharing).
Can a compound have both ionic and covalent bonds?

+
Yes, compounds can exhibit both types of bonding. For example, sodium sulfate (Na2SO4) has an ionic bond between sodium and the sulfate ion, while within the sulfate ion, there are covalent bonds between sulfur and oxygen.