5 Key Differences in Ionic vs. Covalent Bonding Explained
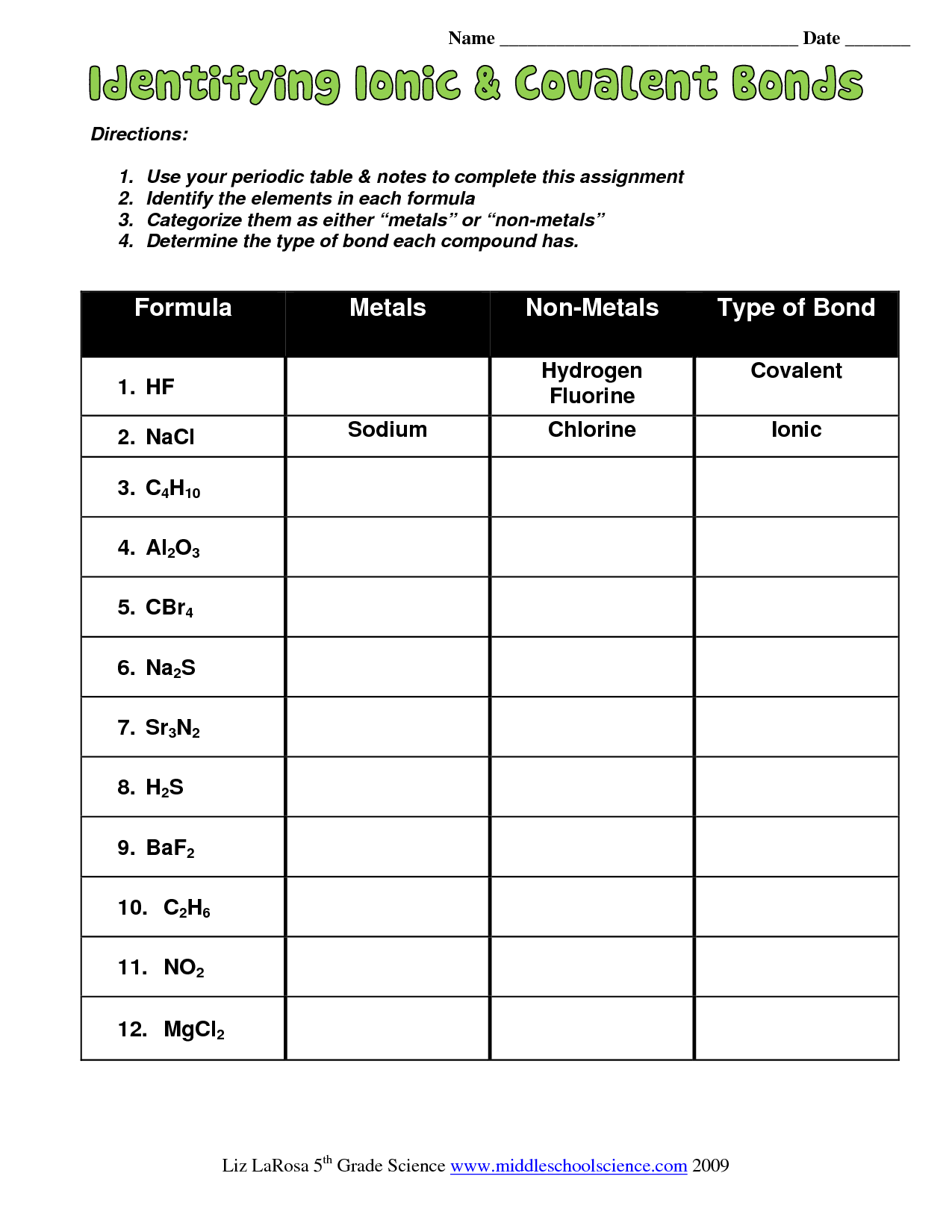
When you dive into the world of chemistry, one of the foundational concepts you'll encounter is the distinction between ionic and covalent bonding. These two types of chemical bonds govern how atoms come together to form compounds, but they do so in fundamentally different ways. Here's an in-depth look at five key differences between ionic and covalent bonding to help clarify this critical chemistry concept.
1. Electron Sharing vs. Transfer

In covalent bonding, atoms share electrons to achieve a stable electron configuration. Here’s how it typically works:
- Two or more atoms combine by sharing one or more pairs of electrons.
- This sharing leads to the formation of molecules, where each atom contributes electrons to a mutual pool.
- Example: Water (H2O), where hydrogen atoms share electrons with oxygen.
Contrastingly, in ionic bonding, there is no electron sharing. Instead:
- An electron is transferred from one atom to another, usually from a metal to a non-metal.
- This transfer results in the formation of positive (cations) and negative (anions) ions.
- Example: Sodium chloride (NaCl), where sodium gives up an electron to chlorine, forming Na+ and Cl- ions.
💡 Note: Covalent bonding often occurs between non-metals, while ionic bonding tends to happen between a metal and a non-metal.
2. Bond Formation and Electronegativity

The difference in electronegativity, or the tendency of an atom to attract electrons, plays a significant role in determining the type of bond formed:
- Covalent bonds: Formed when atoms have similar or small differences in electronegativity, promoting electron sharing.
- Ionic bonds: Occur when there is a large difference in electronegativity, leading to complete electron transfer.
3. Physical Properties

The types of bonds influence the physical properties of the resulting compounds:
| Property | Ionic Bonding | Covalent Bonding |
|---|---|---|
| Melting Point | Generally high due to strong electrostatic forces | Usually low to moderate; depends on molecular structure |
| Solubility | Soluble in polar solvents like water | Can dissolve in both polar and non-polar solvents |
| Conductivity | Conducts electricity when molten or dissolved | Poor conductors unless ionized or polar |
| Brittleness | Brittle due to layer-wise arrangement | Not brittle; can be flexible or soft |

4. Structure and Geometry

The spatial arrangement of atoms in compounds:
- Ionic compounds: Often form crystal lattices, where ions arrange in a repetitive pattern to maximize the attraction between opposite charges.
- Covalent compounds: Exist as discrete molecules with a specific geometry defined by electron pair repulsions (VSEPR theory).
5. Reactivity

The reactivity of compounds with these bonds also differs:
- Ionic: Reactions tend to involve electron exchange or solvent-based ion exchange.
- Covalent: Reactions often involve breaking or forming new covalent bonds, which can be driven by energy considerations, like bond energy.
💡 Note: Polar covalent bonds are a midpoint between ionic and covalent bonds, where electrons are shared unequally.
To sum up, understanding the differences between ionic and covalent bonding is pivotal in comprehending chemical behavior. Ionic bonds are characterized by electron transfer, leading to crystal lattices and properties like brittleness and high melting points. Covalent bonds involve electron sharing, resulting in molecular compounds with varied physical properties depending on their structure. This knowledge isn't just academic; it has practical implications in various fields, from materials science to pharmacology, where bond type influences the strength, solubility, and reactivity of substances.
What makes an ionic bond different from a covalent bond?

+
The primary difference lies in electron interaction. In an ionic bond, electrons are transferred from one atom to another, while in a covalent bond, electrons are shared between atoms.
Why do some elements prefer to form ionic bonds while others form covalent bonds?

+
This preference is largely due to electronegativity. Elements with large differences in electronegativity tend to form ionic bonds, whereas elements with similar electronegativities or small differences prefer covalent bonds.
Can a compound have both ionic and covalent bonds?

+
Yes, many compounds exhibit both types of bonds. For example, in sodium hydroxide (NaOH), the Na and OH- ionize to form an ionic bond, but within the OH- group, the O-H bond is covalent.