Ionic vs Covalent Bonds: Quick Worksheet Guide
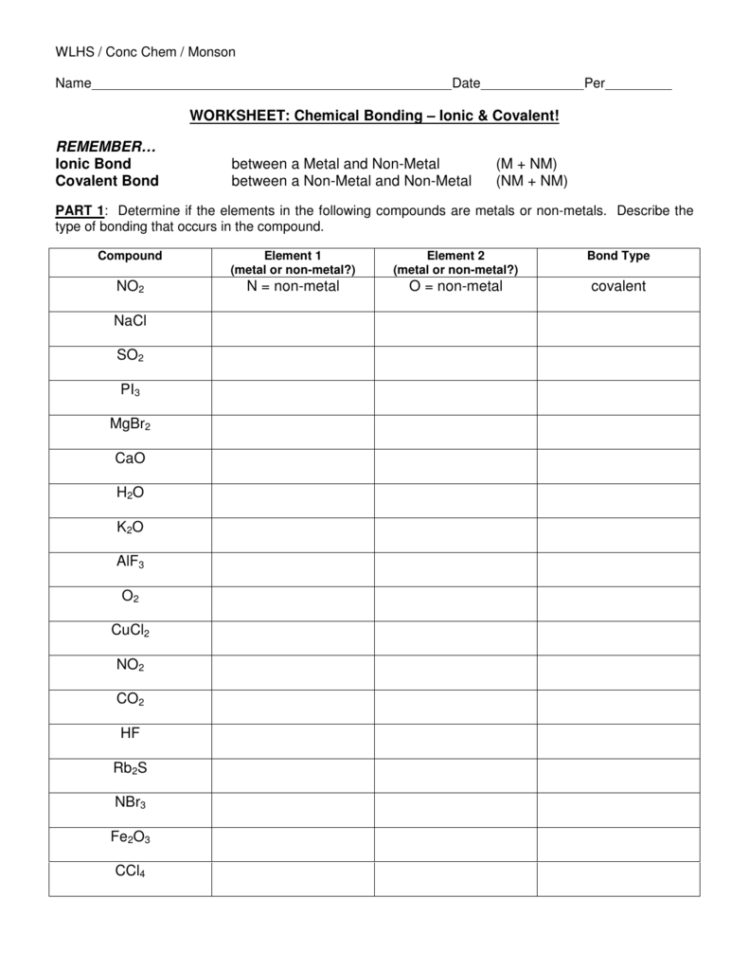
Ionic and Covalent Bonds are fundamental concepts in chemistry that describe how atoms bond to form compounds. Understanding these bonds is crucial for students and professionals alike, as it underpins various chemical reactions and properties of substances. This guide will delve into the distinctions between ionic and covalent bonds, providing a comprehensive comparison and guide for quick reference in worksheets, exams, or projects.
What Are Ionic Bonds?

Ionic bonds occur between atoms when there is a significant difference in electronegativity. Here’s what characterizes an ionic bond:
- Transfer of electrons from one atom to another.
- Formation of ions; one atom loses electrons (cation) while the other gains electrons (anion).
- Electrostatic attraction between oppositely charged ions.
- Compounds formed are typically solids with high melting points.
Examples of Ionic Bonding:

- Sodium Chloride (NaCl): Sodium donates an electron to Chlorine, creating Na+ and Cl-.
- Potassium Fluoride (KF): Here, Potassium loses an electron to Fluorine, forming K+ and F-.
- Magnesium Oxide (MgO): Magnesium gives two electrons to Oxygen, resulting in Mg2+ and O2-.
⚠️ Note: Ionic bonds are not perfectly rigid; at high temperatures, these bonds can be disrupted, leading to melting or vaporization of the compound.
What Are Covalent Bonds?

Covalent bonds form when atoms share electrons to achieve a stable electron configuration. Here are the key characteristics:
- Electrons are shared between atoms rather than transferred.
- They are most commonly observed between elements with similar electronegativities.
- Molecules are formed, which can be nonpolar or polar depending on the electronegativity difference.
- Compounds can exist in various states: solid, liquid, or gas.
Examples of Covalent Bonding:

- Water (H2O): Hydrogen and Oxygen share electrons to form water molecules.
- Ammonia (NH3): Nitrogen and Hydrogen atoms share electrons to create ammonia.
- Methane (CH4): Carbon shares electrons with four Hydrogen atoms to form methane.
💡 Note: In covalent bonds, the distribution of electrons might not be equal, leading to polar covalent bonds where electron density is skewed towards one atom.
Ionic vs. Covalent Bonds: A Side-by-Side Comparison

| Aspect | Ionic Bonds | Covalent Bonds |
|---|---|---|
| Electronegativity Difference | High | Low to Moderate |
| Formation | Transfer of Electrons | Sharing of Electrons |
| State of Compounds | Solid | Solid, Liquid, Gas |
| Melting Point | High | Varies, Generally Lower |
| Electrical Conductivity in Solid State | Non-Conductive | Non-Conductive |
| Electrical Conductivity in Liquid/Melted State | Conductive | Non-Conductive |
| Examples | NaCl, MgO | H2O, CO2 |

Worksheet Tips for Identifying Ionic vs. Covalent Bonds:

Here are some tips for students when identifying and working with these types of bonds in worksheets or exams:
- Look at the electronegativity values of the elements involved. A significant difference indicates an ionic bond.
- Examine the molecular formula. Ionic compounds often have simple, binary formulas (NaCl), while covalent compounds might show multiple types of bonds or even network solids (SiO2).
- Use the periodic table to identify metal-nonmetal or nonmetal-nonmetal interactions.
- Note the physical properties such as melting points and solubility. Ionic compounds tend to have high melting points and dissolve in water to form electrolytes.
Our exploration into ionic and covalent bonds sheds light on how atoms interact to create the complex world of chemical compounds. From the rigidity of ionic lattices to the versatility of molecular formations in covalent bonds, these fundamental concepts allow us to understand the diversity of matter around us. Not only do they govern the behavior of substances at a molecular level, but they also dictate the macroscopic properties we observe in everyday materials.
What is the main difference between ionic and covalent bonds?
+
The primary difference lies in how electrons are handled. Ionic bonds involve the transfer of electrons, creating positive and negative ions that attract each other. Covalent bonds, however, are characterized by sharing electrons between atoms, forming stable electron configurations without the formation of ions.
Can compounds have both ionic and covalent bonds?
+
Yes, many compounds display both types of bonding. A classic example is sodium hydroxide (NaOH), where the bond between Na+ and OH- is ionic, while the O-H bond within the hydroxide ion is covalent.
Why do ionic compounds generally have high melting points?

+
Ionic compounds have high melting points due to the strong electrostatic forces of attraction between the ions in the lattice. It takes a significant amount of energy to break these bonds and disrupt the lattice structure.