Ion Formation Worksheet Answers
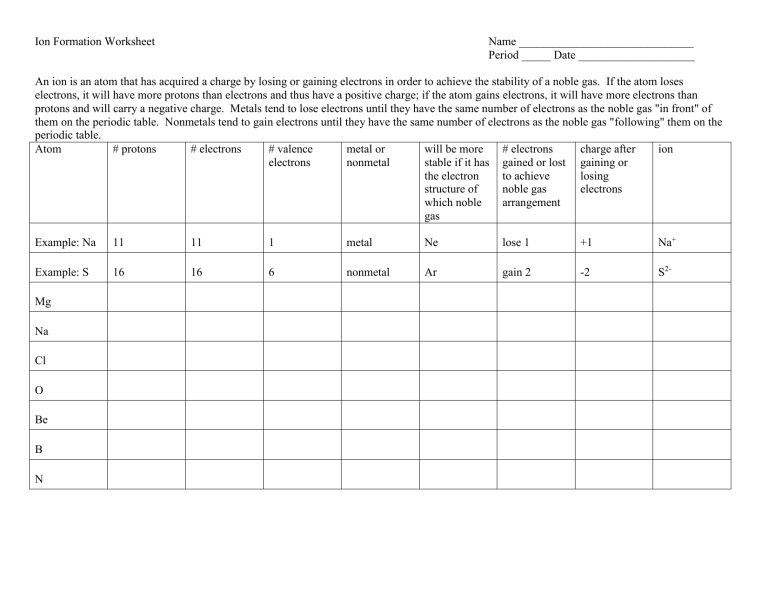
Understanding how ions form and behave is fundamental to grasping chemistry at a basic level. For students and educators alike, knowing how to work through ion formation worksheets can illuminate many aspects of chemical interactions and reactions. This blog post aims to provide detailed answers and explanations for common ion formation worksheet questions, enhancing comprehension and application of this concept.
What are Ions?
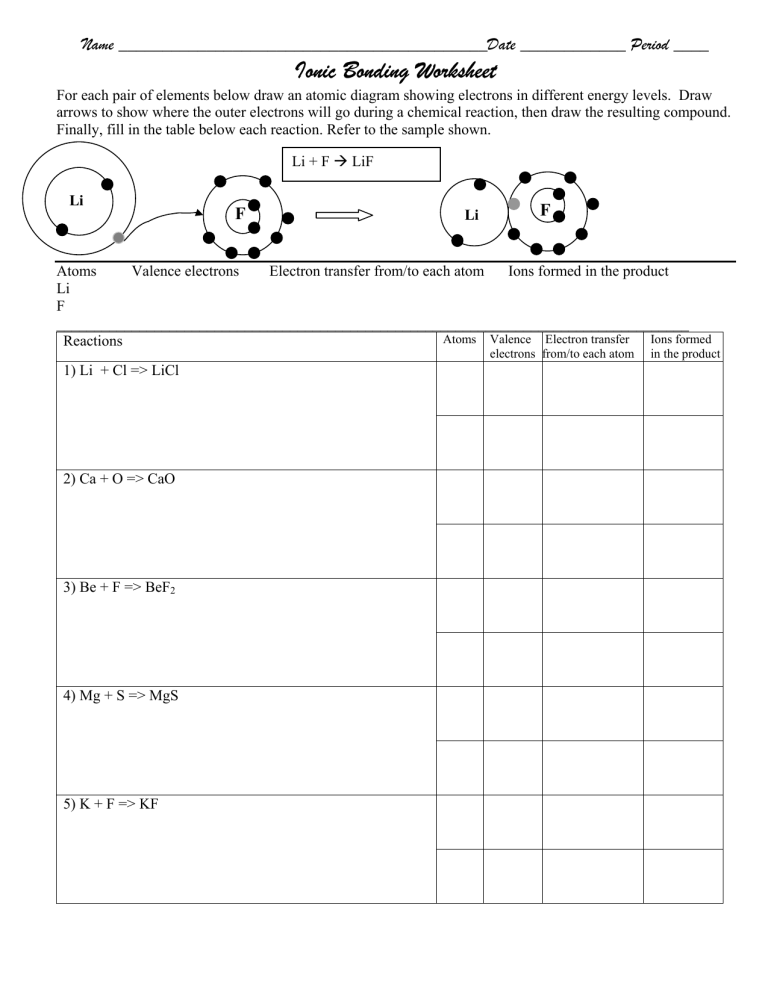
Before we dive into the worksheet, let's define what ions are. An ion is an atom or molecule that has lost or gained one or more electrons, giving it a net electrical charge. Here's a brief overview:
- Cations: Positively charged ions. They are formed when an atom loses electrons.
- Anions: Negatively charged ions, formed when an atom gains electrons.
Common Worksheet Questions and Answers
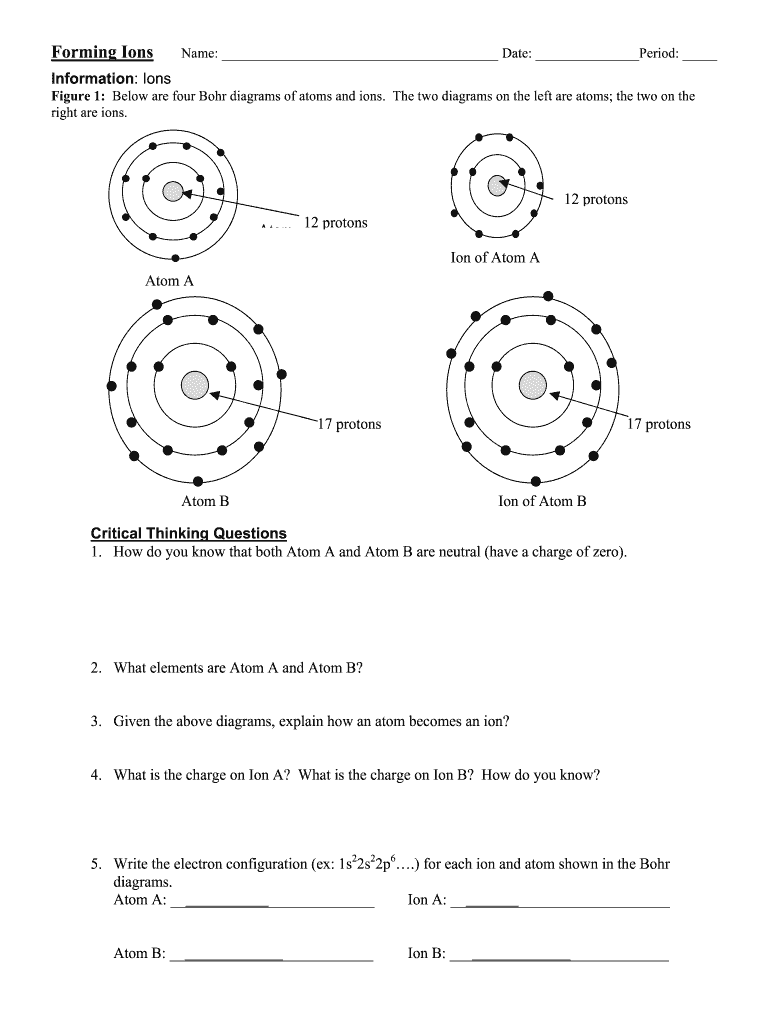
Question 1: How do elements form ions?
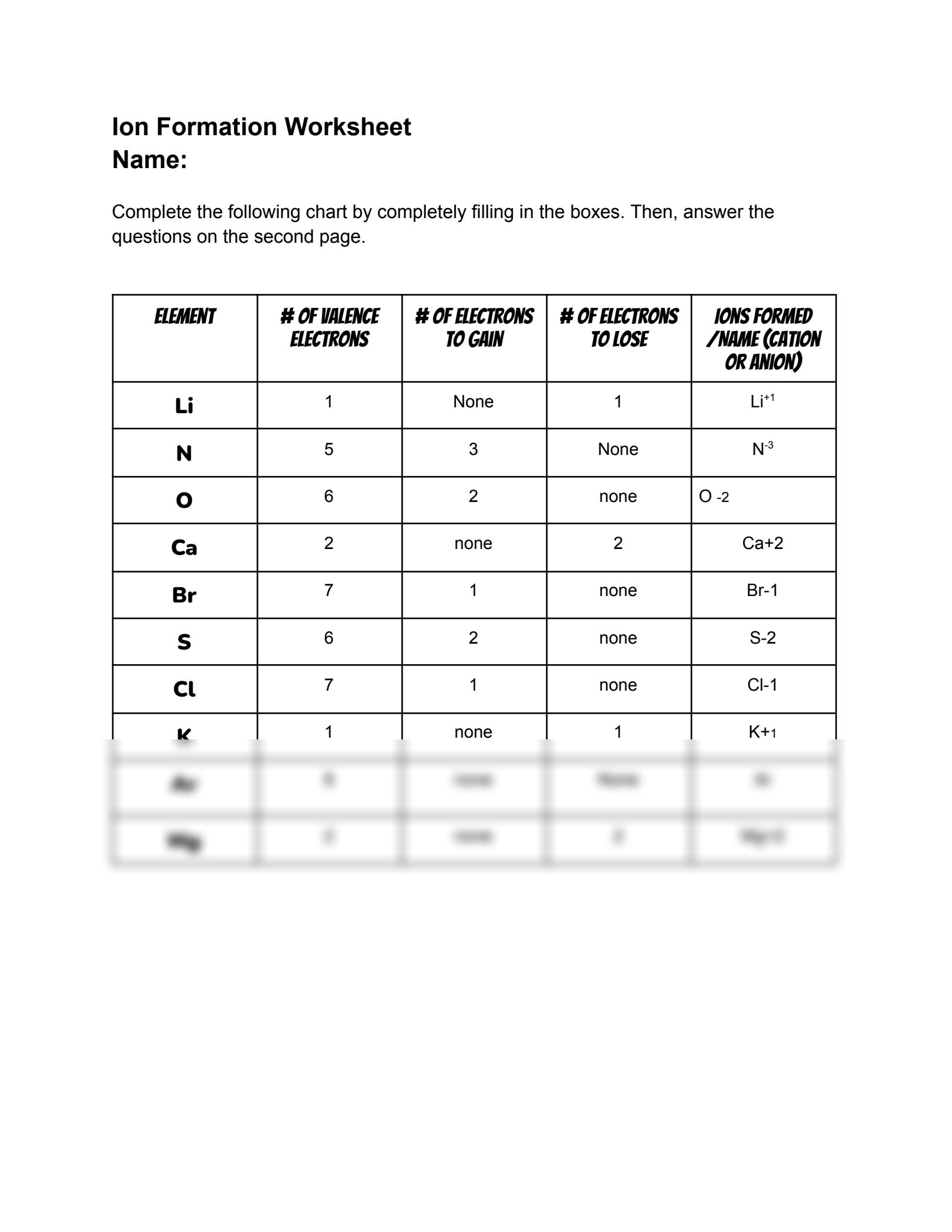
Elements form ions to achieve a stable electron configuration, usually one that mimics the inert noble gases, which have full outer electron shells. Here’s how:
- Metals (like sodium or potassium) typically lose electrons to become positive ions (cations).
- Non-metals (such as chlorine or oxygen) tend to gain electrons to form negative ions (anions).
Question 2: Predict the charge of an ion from the periodic table.

One can often predict an element’s ionic charge based on its position in the periodic table:
| Group | Ionic Charge |
|---|---|
| 1 (Alkali Metals) | +1 |
| 2 (Alkaline Earth Metals) | +2 |
| 13 (Boron Group) | +3 (common for metals like Aluminum) |
| 15 (Nitrogen Group) | -3 |
| 16 (Chalcogens) | -2 |
| 17 (Halogens) | -1 |
| 18 (Noble Gases) | 0 (stable, rarely form ions) |

⚠️ Note: Transition metals often have multiple possible charges, which can’t be predicted as easily from the periodic table alone.
Question 3: Identify and name the following ions:

- Na+ - Sodium cation.
- Cl- - Chloride anion.
- O2- - Oxide anion.
- Fe2+ - Iron(II) or ferrous cation.
- Fe3+ - Iron(III) or ferric cation.
🌟 Note: The use of Roman numerals for transition metals indicates the oxidation state or charge on the ion.
Question 4: What happens to the number of protons and electrons in ion formation?
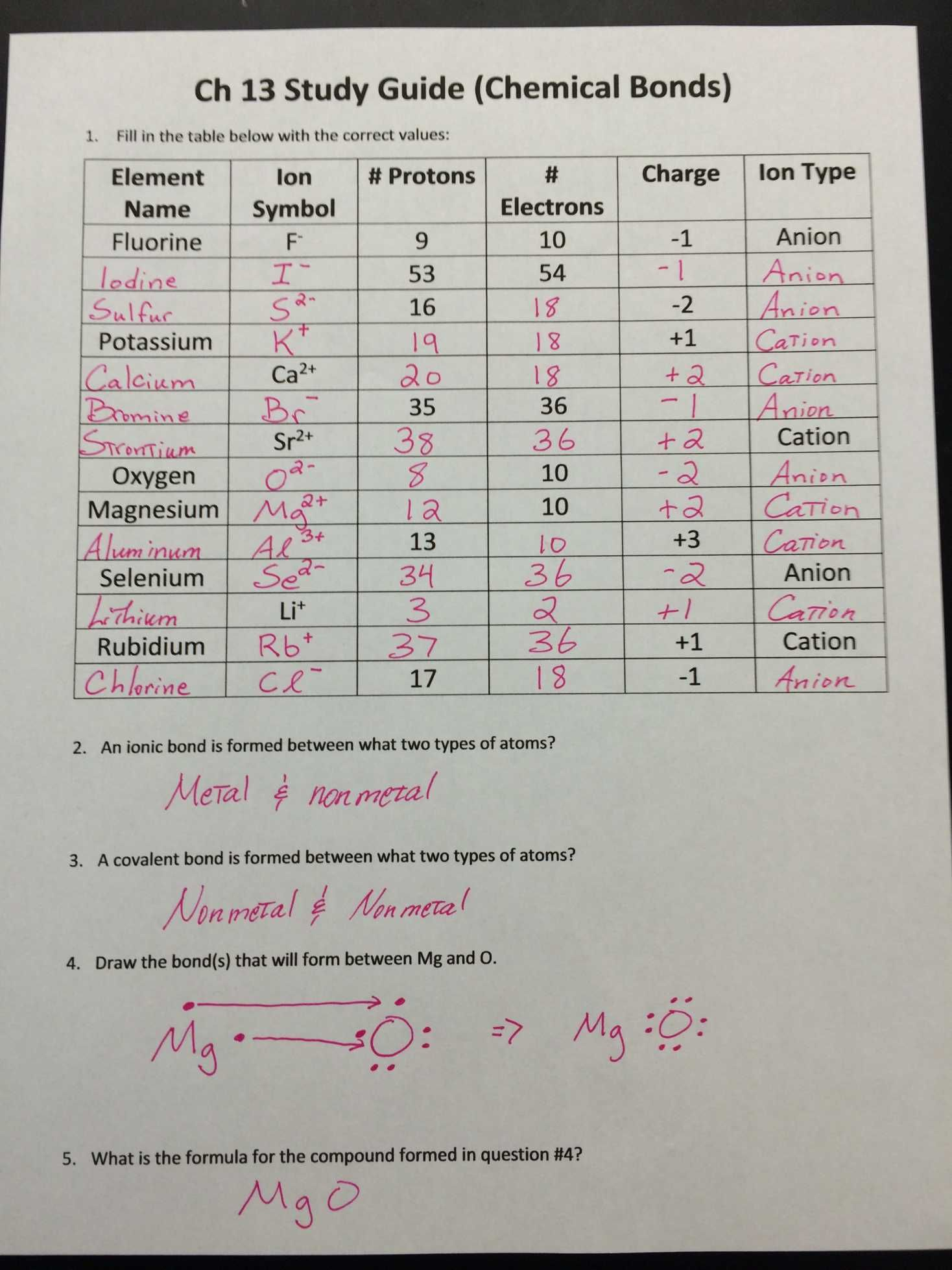
When an atom forms an ion:
- The number of protons remains unchanged, as protons are part of the nucleus.
- The number of electrons changes:
- If electrons are lost, the ion has fewer electrons than protons, resulting in a positive charge.
- If electrons are gained, the ion has more electrons than protons, resulting in a negative charge.
Question 5: Explain the difference between monatomic and polyatomic ions.

Monatomic ions are single atoms with a charge, like Na+ or Cl-. Polyatomic ions, on the other hand, consist of two or more atoms covalently bonded together with a net charge, such as:
- OH- - Hydroxide ion
- NO3- - Nitrate ion
- SO42- - Sulfate ion
This overview covers the basics of ion formation, charges, and identification. Understanding these principles is crucial for anyone studying chemistry or related fields. The concept of ion formation not only helps in predicting chemical behavior but also underpins many practical applications from energy storage (like batteries) to understanding biological systems where ions play a critical role.
Why do some elements form multiple ions?

+
Some elements, especially transition metals, can form multiple ions because they have unfilled d-orbitals which allow for the loss of different numbers of electrons.
Can atoms lose or gain more than one electron?

+
Yes, atoms can lose or gain multiple electrons. The number of electrons lost or gained depends on the electron configuration and energy levels available to achieve stability.
What is the significance of ions in daily life?
+
Ions play key roles in many everyday phenomena, from battery operation and water purification to the human body’s functioning where electrolytes (ions in solution) are vital for hydration, nerve impulse transmission, and muscle function.