Periodic Table Basics: A Beginner's Worksheet Guide
Embarking on a journey through the fascinating world of chemistry begins with understanding the Periodic Table, a cornerstone tool for chemists and students alike. The Periodic Table is not just a chart of elements but a roadmap to understanding how the universe is built. In this post, we'll provide an introductory Periodic Table Worksheet Guide that demystifies this crucial tool, helping beginners to visualize, memorize, and apply the knowledge in practical chemistry. From the basics of element groups to trends and patterns, we'll navigate you through step-by-step learning to master the Periodic Table.
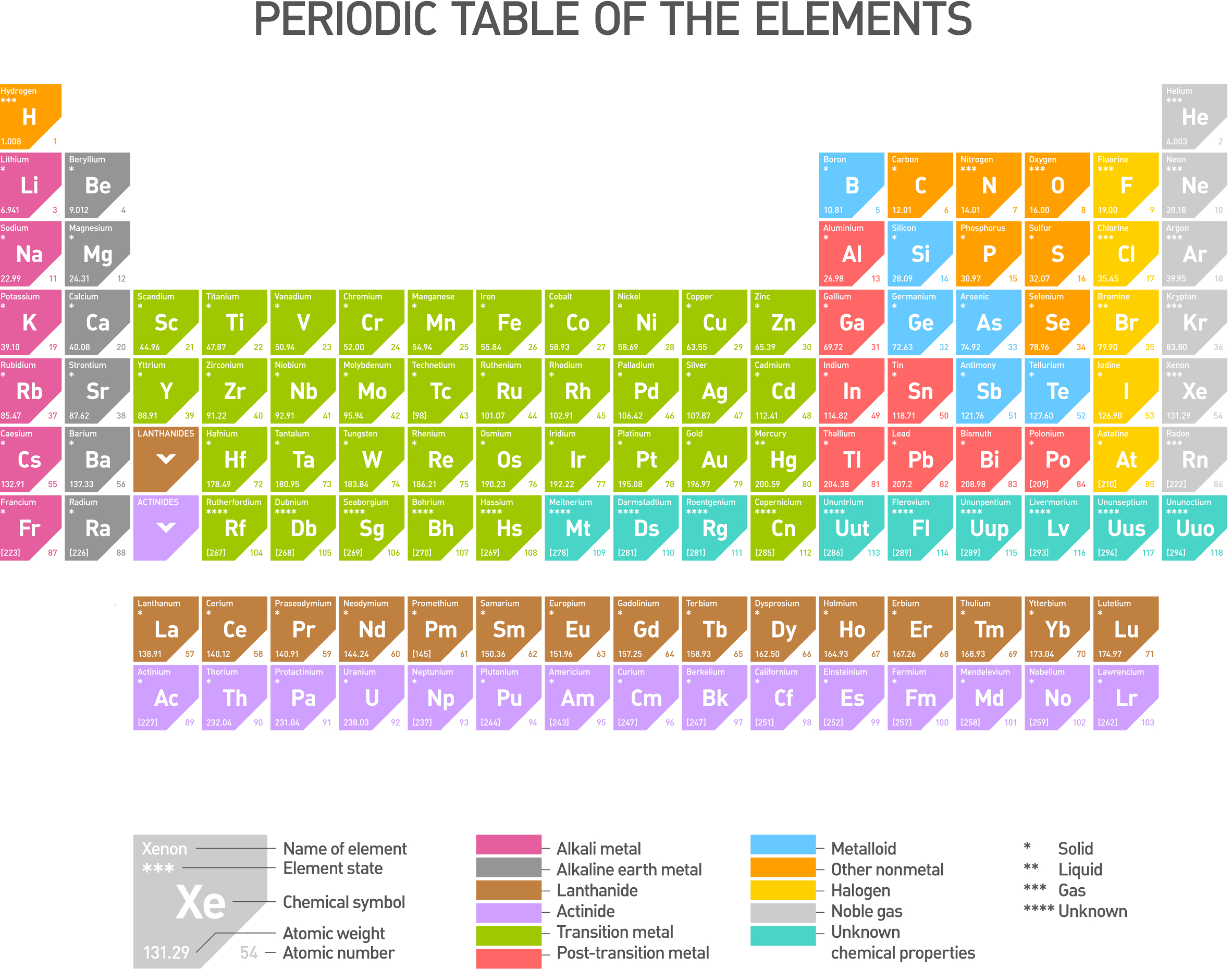
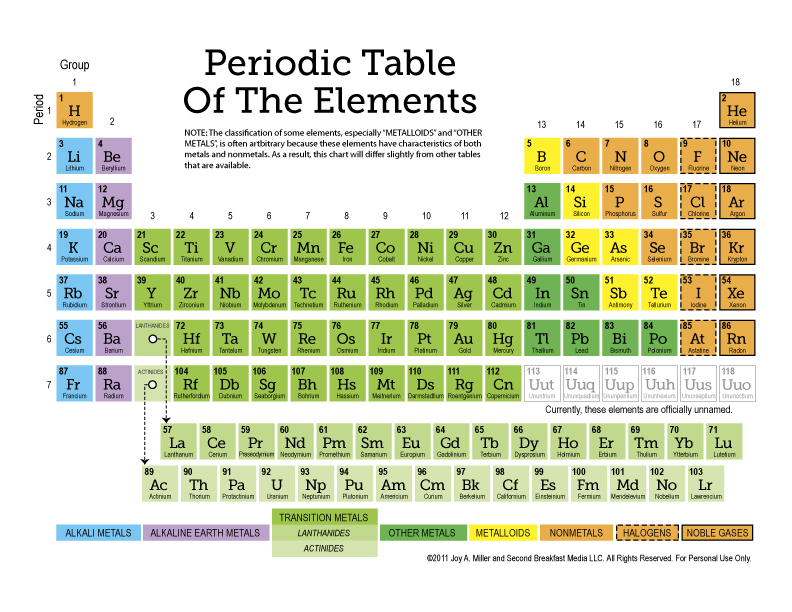
Understanding the Structure of the Periodic Table

The Periodic Table organizes elements in a grid-like fashion, with elements arranged according to their atomic number, electron configurations, and recurring chemical properties.
Reading the Table

- Rows (Periods): Each horizontal row indicates the number of energy levels or shells surrounding the nucleus of an atom.
- Columns (Groups): Elements in the same vertical column share similar chemical behavior due to the same number of valence electrons.
- Atomic Number: This number indicates the number of protons in an element’s nucleus and is used to order elements.
- Element Symbol: Often a shorthand of the element’s name, usually derived from Latin or Greek origins.
- Element Name: The full name of the element.
- Atomic Mass: The average mass of an element’s isotopes, taking into account their natural abundance.

Key Areas of the Table

- Alkali Metals: Located in Group 1, excluding Hydrogen, these metals are highly reactive due to their single valence electron.
- Alkaline Earth Metals: Found in Group 2, they are slightly less reactive than alkali metals but still form stable ionic compounds.
- Transition Metals: These occupy the middle block, known for their variable oxidation states and metallic properties.
- Metalloids: Elements on the staircase line, exhibiting properties of both metals and non-metals.
- Non-Metals: Generally to the right of the staircase line, they tend to be insulators or semiconductors.
- Halogens: In Group 17, these non-metals are highly reactive due to their desire to gain an electron.
- Noble Gases: Group 18 elements with full electron shells, making them inert or “noble.”
⚠️ Note: Hydrogen, while typically placed above the alkali metals, is somewhat unique and doesn’t fit neatly into any group.
Practical Applications of the Periodic Table
Now that we’ve covered the basics, let’s dive into how to use this knowledge practically:
Memorizing Elements

- Focus on the first 20 elements, which are the most frequently encountered.
- Use mnemonic devices or songs to remember the sequence.
- Create flashcards or use apps for repeated revision.
Element Trends

- Atomic Radius: Increases from right to left and down each group.
- Electronegativity: Decreases down a group and increases from left to right across a period.
- Ionization Energy: Similar trend to electronegativity, higher for elements with higher electron affinities.
| Property | Trend Across Periods | Trend Down Groups |
|---|---|---|
| Atomic Radius | Decreases | Increases |
| Electronegativity | Increases | Decreases |
| Ionization Energy | Increases | Decreases |
Element Reactivity

- Metals on the left are more reactive, with the alkali metals being the most reactive group.
- Non-metals on the right become more reactive as you move towards the halogens.
📌 Note: The reactivity of elements is a key concept for understanding chemical reactions and balancing equations.
Valence Electrons and Bonding

Knowing the number of valence electrons helps predict how elements will bond:
- Elements with the same number of valence electrons often have similar properties.
- Octet rule: Most elements strive to achieve a noble gas configuration through bonding.
- Ionic bonding involves the transfer of electrons, covalent bonding involves sharing.
Worksheet Exercises

Here’s a worksheet guide to practice and solidify your understanding of the Periodic Table:
Exercise 1: Elements Identification

- Identify elements based on their atomic number, mass, and symbol.
- Practice grouping elements by periods and groups.
Exercise 2: Trend Analysis

- Plot trends like atomic radius, electronegativity, and ionization energy across a row or down a column.
Exercise 3: Chemical Properties and Reactivity

- Predict the behavior of elements based on their group and position in the table.
- Create simple compounds or ions and identify their charges.
Exercise 4: Electron Configurations

- Work out the electron configurations of different elements.
- Determine the valence electrons and predict bonding behavior.
In this comprehensive guide, we've explored the Periodic Table's structure, how to read and understand it, and some key applications in practical chemistry. Through a blend of theory and interactive worksheets, you now have the tools to not only navigate the Periodic Table but also to predict chemical behavior, trends, and bonding patterns. Remember that learning chemistry involves continuous practice, so engage with these exercises regularly to deepen your understanding. Whether you're a student looking to improve your grades or a hobbyist wanting to understand the building blocks of nature, mastering the Periodic Table is a significant step towards scientific literacy and unlocking the secrets of matter.
Why is the Periodic Table arranged in the order it is?
+
The Periodic Table is arranged by increasing atomic number, which reflects the number of protons in an element’s nucleus. Elements are grouped in such a way that elements with similar properties fall into the same columns (groups), mainly due to their electron configuration and valence electron count.
What are valence electrons and why are they important?
+
Valence electrons are the outermost electrons of an atom and are crucial because they determine an element’s chemical behavior, its ability to bond with other elements, and its reactivity.
How do I remember the Periodic Table?
+
Memory aids like songs, mnemonics, flashcards, or associating elements with their uses in daily life can make learning the Periodic Table easier. Regular practice and repetition are key.