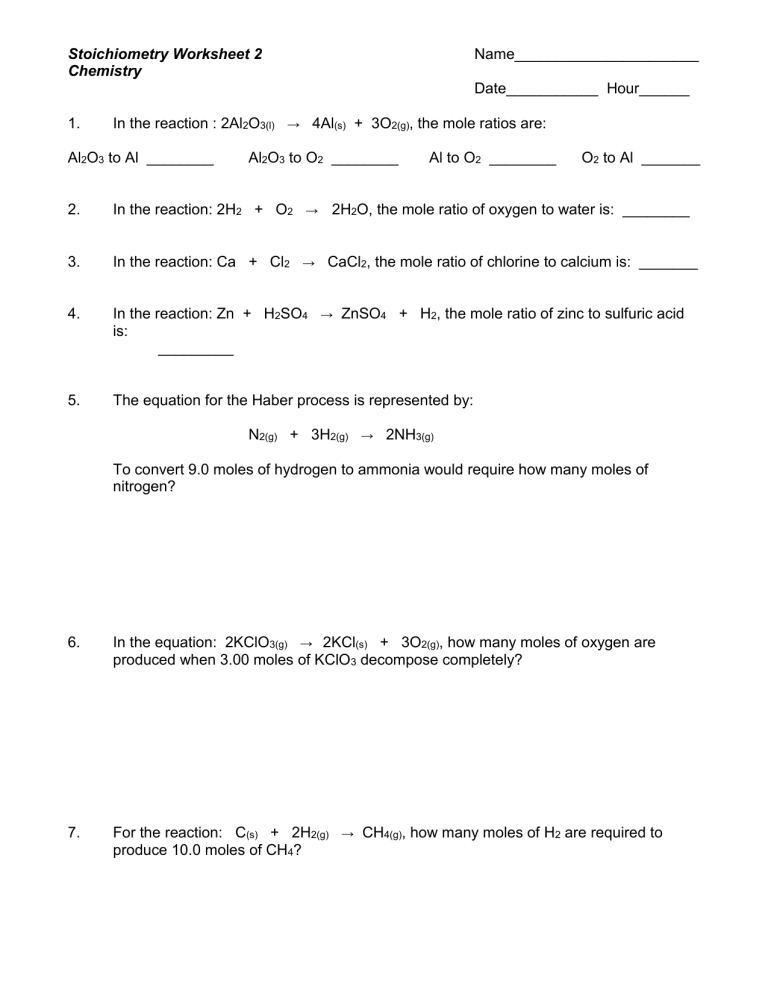
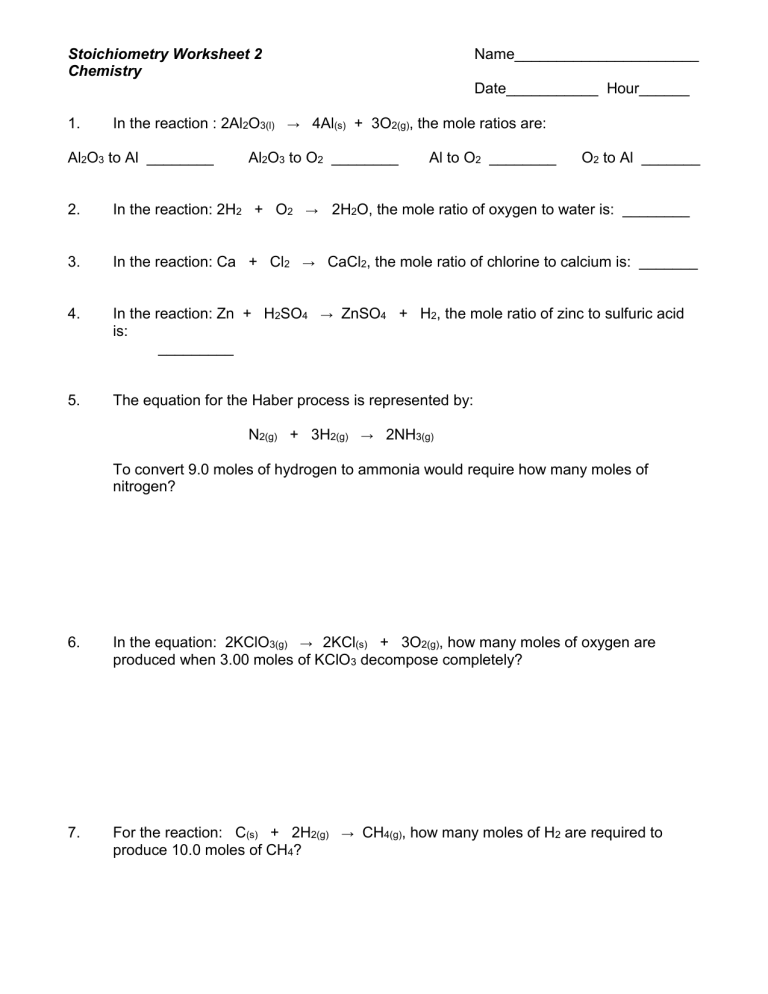
Intro To Stoichiometry Worksheet Answers

Stoichiometry is an essential concept in chemistry, particularly when dealing with chemical reactions and balancing equations. This blog post aims to explore stoichiometry worksheet answers, providing students with a comprehensive guide to understanding and solving stoichiometric problems. Whether you're calculating mole ratios, determining limiting reactants, or predicting reaction yields, stoichiometry lays the groundwork for a deeper understanding of chemical processes.
Understanding Stoichiometry

Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Here’s a brief overview:
- Mole-Mole Relationships: Understand how the mole concept can predict the ratio in which substances react or are produced.
- Mass-Mass Calculations: Use the molar mass to convert from mass of reactants to the mass of products or vice versa.
- Limiting Reactants: Determine which reactant is used up first, thereby stopping the reaction, to find the maximum amount of product that can be made.
- Percent Yield: Calculate the efficiency of a reaction by comparing the actual yield to the theoretical yield.

Sample Problems and Answers

Let’s delve into some practical examples:
1. Mole-Mole Relationships
Given the balanced equation: 2H2 + O2 → 2H2O
- How many moles of H2O are produced if 3 moles of H2 are reacted?
Since the mole ratio of H2 to H2O is 2:2 (which simplifies to 1:1), 3 moles of H2 will produce 3 moles of H2O.
2. Mass-Mass Calculations

Using the same equation:
- If 4 grams of H2 react completely, how many grams of H2O will be produced?
First, find moles of H2 (4 g / 2 g/mol = 2 mol). From the mole ratio, 2 moles of H2 produce 2 moles of H2O. Convert this to grams:
2 mol H2O × 18 g/mol = 36 grams of H2O.
3. Limiting Reactant

Consider the reaction: N2 + 3H2 → 2NH3
- If you have 10 moles of N2 and 30 moles of H2, what is the limiting reactant and how much NH3 can be produced?
From the mole ratio:
1 mole N2 produces 2 moles NH3.
3 moles H2 produce 2 moles NH3.
N2 is the limiting reactant because it can only produce 20 moles of NH3, whereas H2 can produce up to 40 moles of NH3.
4. Percent Yield

If the reaction theoretically yields 20 moles of NH3 but you only collect 15 moles in your experiment:
- What is the percent yield?
Percent yield = (Actual yield / Theoretical yield) × 100 = (15 / 20) × 100 = 75% yield.
🌟 Note: Always double-check your units to ensure your calculations are consistent.
Common Mistakes in Stoichiometry

- Not balancing the chemical equation before starting calculations.
- Ignoring the stoichiometric ratio and using just the coefficients directly from the equation.
- Forgetting to convert mass to moles or vice versa when working with mass-mass calculations.
- Misidentification of the limiting reactant due to overlooking the mole ratio.
| Type of Calculation | Common Pitfalls |
|---|---|
| Mole-Mole | Not recognizing the coefficients as mole ratios. |
| Mass-Mass | Failure to convert mass to moles correctly. |
| Limiting Reactant | Miscalculating the amount of products based on ratios. |
| Percent Yield | Not considering both actual and theoretical yield. |

📝 Note: Use dimensional analysis to track units and ensure all conversions are correct.
Wrapping Up

The realm of stoichiometry is both fascinating and crucial for understanding how substances react with one another at a molecular level. We’ve explored mole-mole relationships, mass-mass calculations, identification of limiting reactants, and the calculation of percent yield, providing you with answers to common stoichiometry problems. This knowledge not only aids in problem-solving but also fosters a deeper appreciation of the quantitative nature of chemical reactions.
What is stoichiometry used for in real-life scenarios?

+
Stoichiometry is fundamental in industries like pharmaceuticals, where exact quantities of reactants are crucial for producing medicines with consistent quality. Environmental science also uses stoichiometry to predict how much of a pollutant can be neutralized by a given reagent.
Can stoichiometry be applied to both chemical and physical changes?
+
While stoichiometry is primarily associated with chemical reactions, it can indeed be applied to some physical changes, like phase transitions, where the concept of moles and conservation of matter still applies.
What does a 100% yield signify in a chemical reaction?

+
A 100% yield theoretically means that all reactants have been converted into products with no loss. However, in real-world scenarios, achieving 100% yield is rare due to side reactions, incomplete reactions, or losses during purification processes.
How do you know if a problem requires stoichiometry?

+
Any problem where you need to determine how much of one substance will react or be produced given a certain amount of another substance usually involves stoichiometry. Look for cues like “how many moles,” “what mass,” “limiting reactant,” or “yield” in the question.
What is the importance of balancing chemical equations in stoichiometry?

+
Balancing equations is critical because it ensures that the law of conservation of mass is obeyed, reflecting the correct stoichiometric ratios between reactants and products. Without a balanced equation, any stoichiometric calculation would be fundamentally flawed.